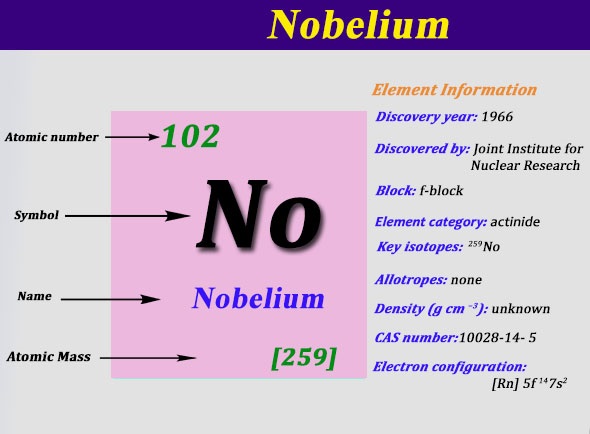
Nobelium Electron Configuration: Nobelium is a synthetic chemical element that has a symbol No. The atomic number of Nobelium is 102. It is named to give the honor to Alfred Nobel, the one who invented dynamite and benefactor of science. It is a radioactive metal and the tenth transuranic element.
- Hydrogen Electron Configuration
- Helium Electron Configuration
- Lithium Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
Nobelium is the penultimate member of the actinide series. Nobelium can only be produced in particle accelerators by bombarding lighter elements with charged particles, Like all elements with an atomic number over 100. A total of twelve nobelium isotopes are known to be existing.
- Hydrogen Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Oxygen Valence Electrons
- Fluorine Valence Electrons
- Neon Valence Electrons
- Sodium Valence Electrons
- Magnesium Valence Electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Phosphorus Valence Electrons
- Sulfur Valence Electrons
- Chlorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Iron Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
- Germanium Valence Electrons
- Arsenic Valence Electrons
- Selenium Valence Electrons
- Bromine Valence Electrons
- Krypton Valence Electrons
- Rubidium Valence Electrons
- Strontium Valence Electrons
- YttriumValence Electrons
- Zirconium Valence Electrons
- Niobium Valence Electrons
- Molybdenum Valence Electrons
- Technetium Valence Electrons
- Ruthenium Valence Electrons
- Rhodium Valence Electrons
- Palladium Valence Electrons
Nobelium Electron Configuration
The most stable isotope of nobelium is 259No that has a half-life of 58 minutes, but the shorter-lived isotope that is 255No (half-life 3.1 minutes) is most commonly used in chemistry as it can be produced on a larger scale.
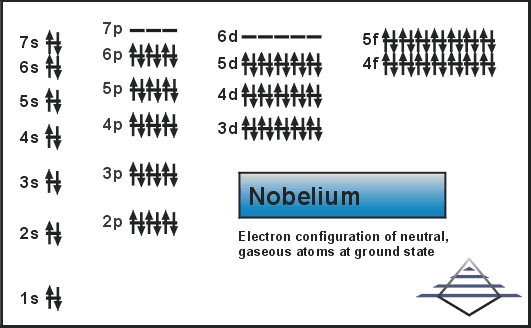
Today we are going to share the information about the electron configuration of the Nobelium along with the pictures highlighting the details. If you are also here to get the information about the electron configuration of the Nobelium then you are at the right place. If you are a student or a teacher then it will help you a lot. For more information please see the full post below.
What is the Electron Configuration of Nobelium
Rn 5f14 7s2 is the electron configuration of the Nobelium.
How Many Valence Electrons Does Nobelium Have
Nobelium has four valence electrons in its outer shell.
Nobelium Number of Valence Electrons
There are four valence electrons in the outer shell of the nobelium.
Leave a Reply