Nitrogen Valence Electrons: As you know, valency is the potential of an element to combine or displace the electrons of atoms of another element. It the number of electrons an electron needs to achieve to reach stability or inert gas octet state. So today we will discuss What Is Valency of Nitrogen.
- Hydrogen Valency
- Helium Valency
- Beryllium Valency
- Boron Valency
- Fluorine Valency
- sodium Valency
- Aluminum Valency
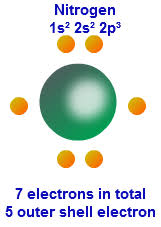
Nitrogen Valence Electrons Dot Diagram
How many valence electrons does Nitrogen have?
Nitrogen has 5 electrons in its outer shell and 2 electrons in its inner shell. Hence it has a total of 7 electrons. The valency of nitrogen depends upon which nitrogen ion is in question. An N+1 ion has a valency of 6 (2+4), N2+ has 5 (2+3), N3+ has 4 electrons (2 core electrons and 2 valence electrons). If you want to check out the position of the Nitrogen element in the periodic table then you should cross-check the homepage of this site.
What Is The Valency Of Nitrogen In Ammonium
The valency of Nitrogen in Ammonia is 3. The number of electrons shared by nitrogen with hydrogen in ammonia is 3 to complete its octet state. The remaining 2 electrons remain as single electrons and do not pair with other electrons.
Leave a Reply