Chlorine Valence Electrons: Earlier, we have discussed the valency of many important elements. It is important for any science student to know the valency of these important elements since they are essential to understanding many important concepts of chemistry. So today we shall discuss How Many Valence Electrons Are There in Chlorine?
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
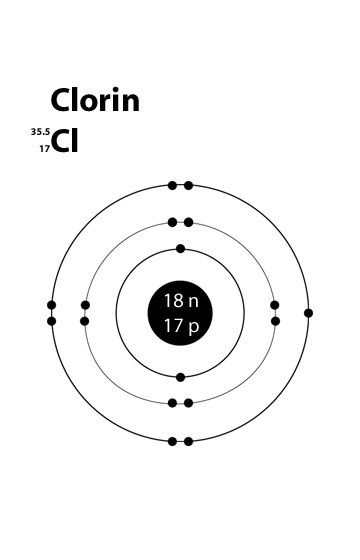
Chlorine Valence Electrons Dot Diagram
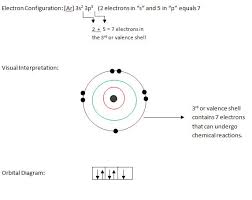
The atomic number of chlorine is 17. Hence it has 2 electrons in its innermost shell, 8 electrons in its second shell and 7 electrons electron in the outer-most shell respectively.
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- iron Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
- Germanium Valence Electrons
- Arsenic Valence Electrons
- selenium Valence Electrons
How many valence electrons does chlorine have?
Since it has 7 electrons in its outer-most shell, it needs one more electron to achieve stability or the octet state. Since it needs to add another electron by combining with the atom of another element, it has got a valency of -1. Check out the periodic table on the homepage. Chlorine is a very popular gas.
The number of valence electrons of Cl have been shown in this given picture.
Valence Electron of Chlorine
The atomic number of chlorine is 17. Hence it has got 7 electrons in its outermost shell. Its valency can be found out by subtracting 7 from 8, i.e., -1.
Leave a Reply