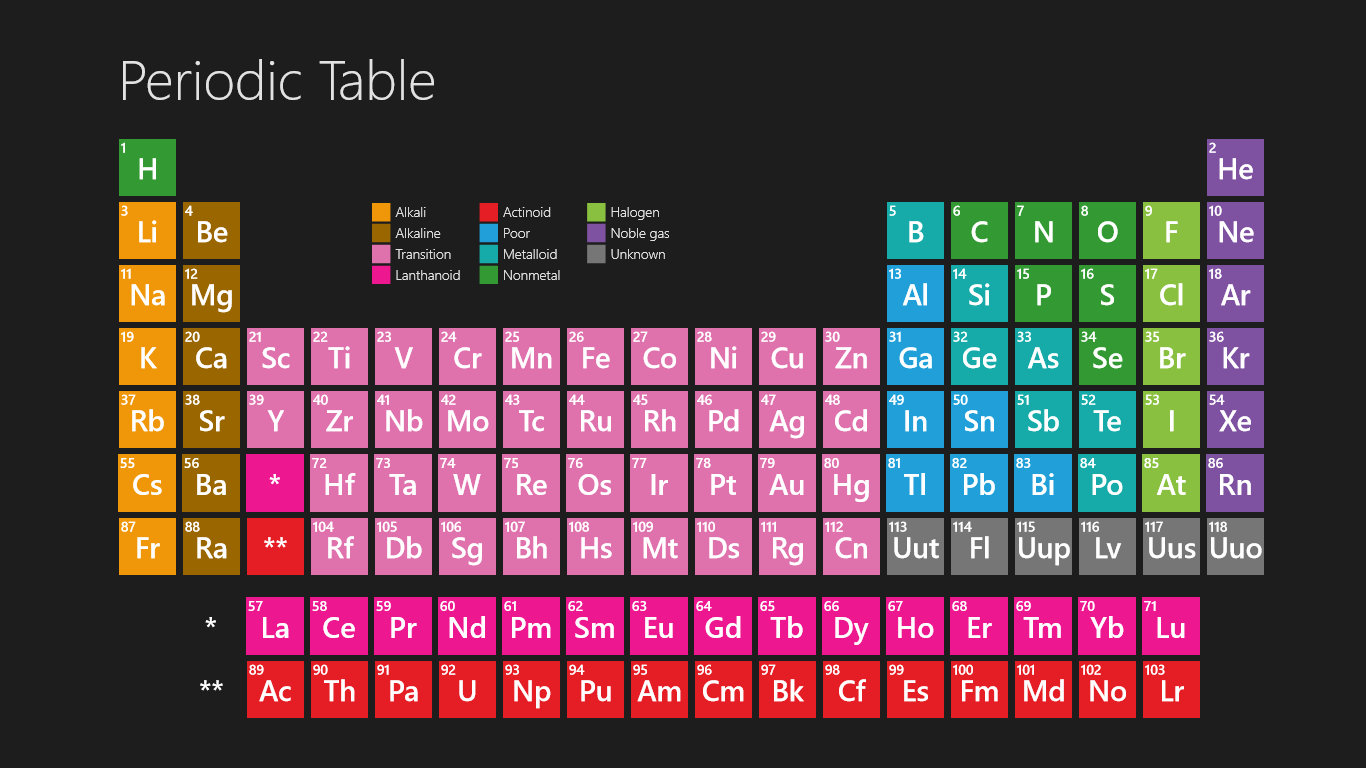
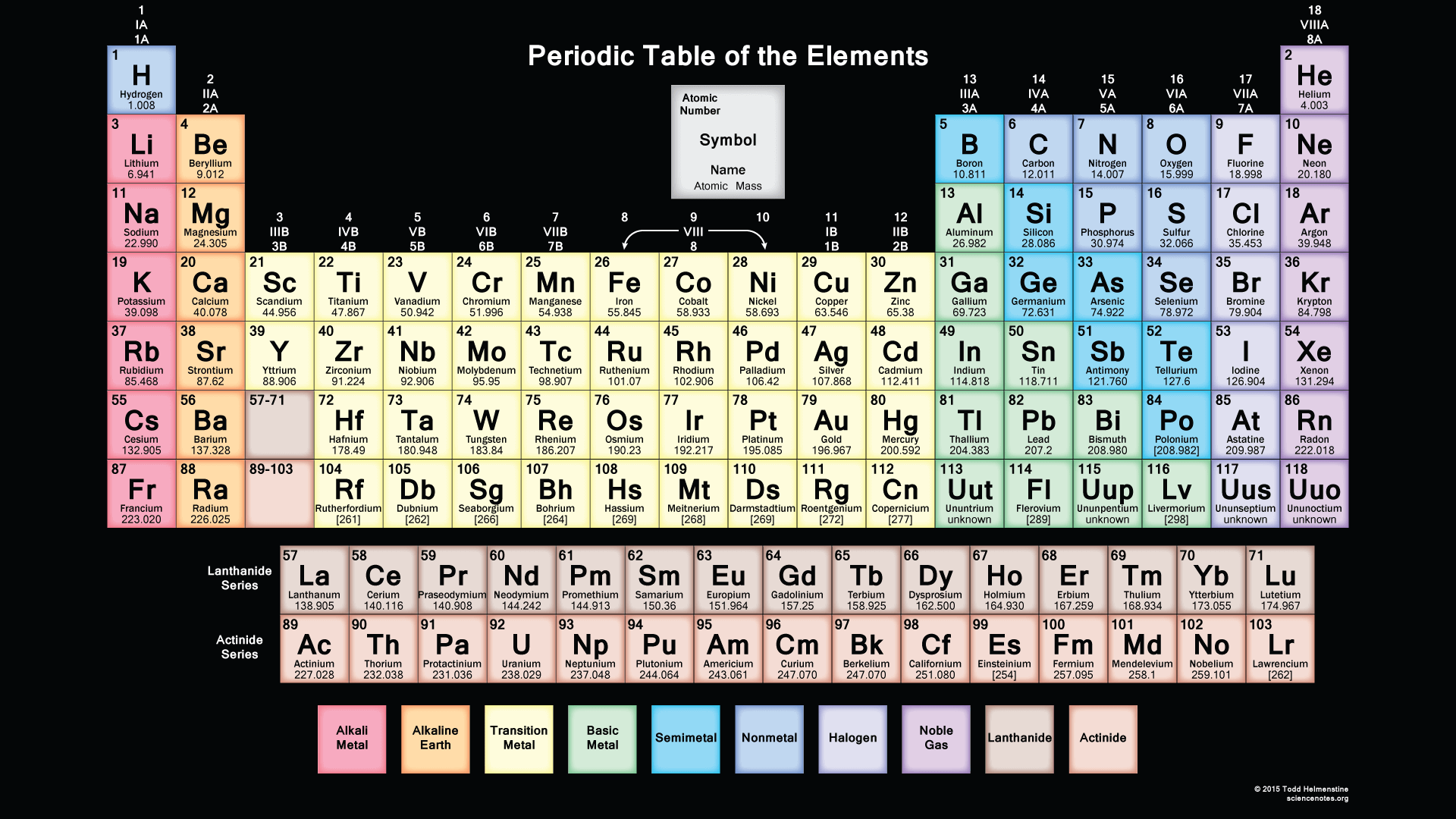
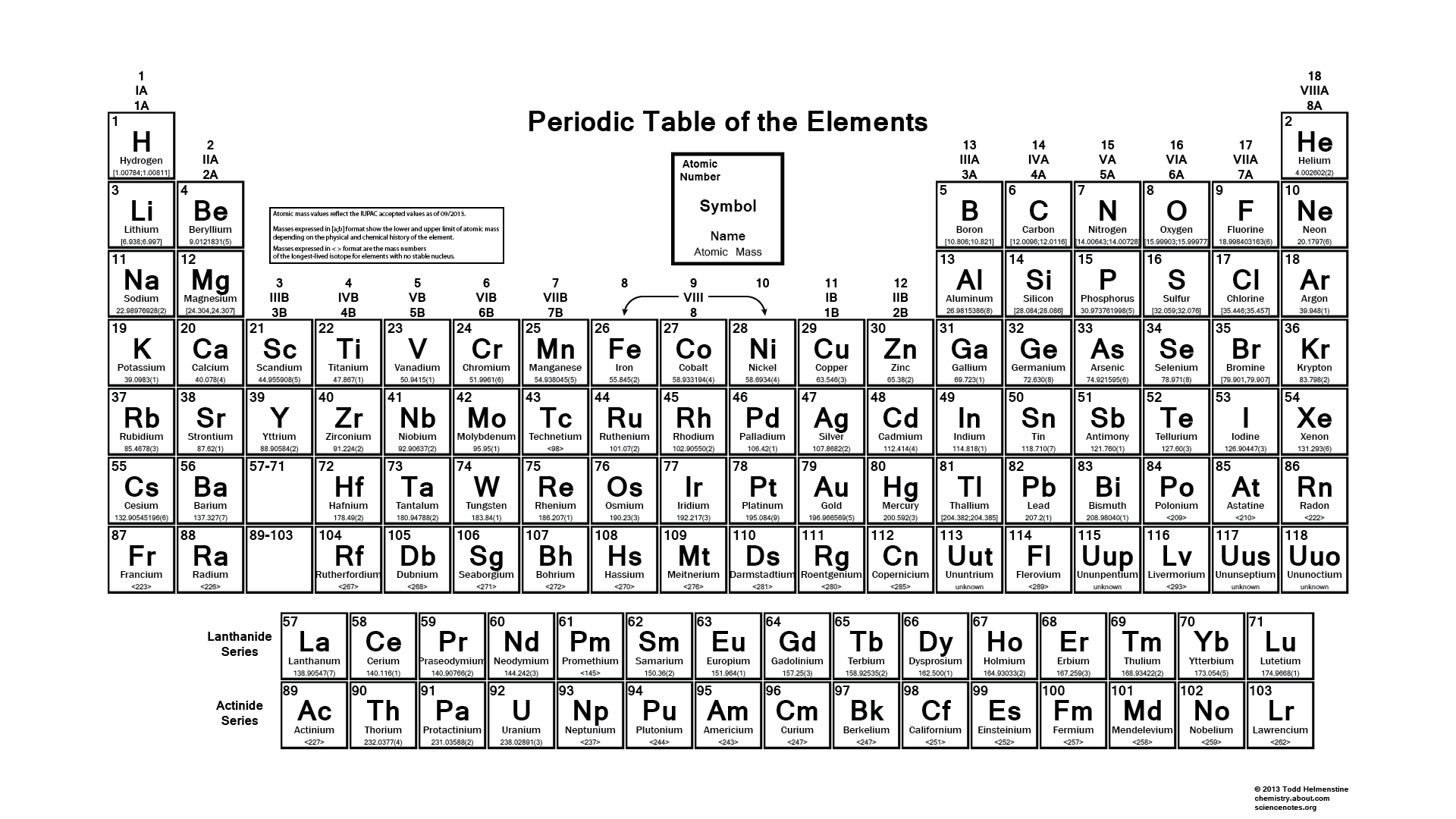
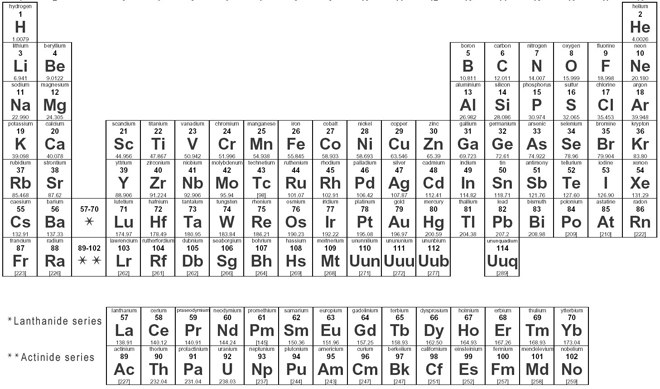
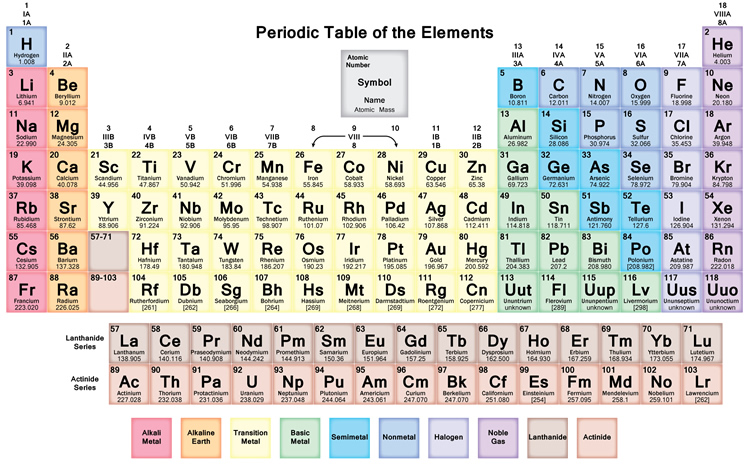
We searched for Periodic Table or P Table but we didn’t get the complete information regards the periodic table but don’t need to worry here you get complete information regarding the periodic table in a PDF file which you can easily save on your device and then use it. What we have done on our end is that we have to make a list with some important links so that you get all the information.
The HD periodic table for kids helps you a lot get clear information which you can see on your device also. You can use it and fit it on your sheet also and don’t need to take tension regarding if the paper is lost then what, so friends don’t worry about that because you can also take print as much as you can of this periodic table for your personal use where you take it for homework problems or performing lab calculations.
- Molybdenum Valence Electrons
- Technetium Valence Electrons
- Ruthenium Valence Electrons
- Rhodium Valence Electrons
- Palladium Valence Electrons
Free Printable Periodic Table
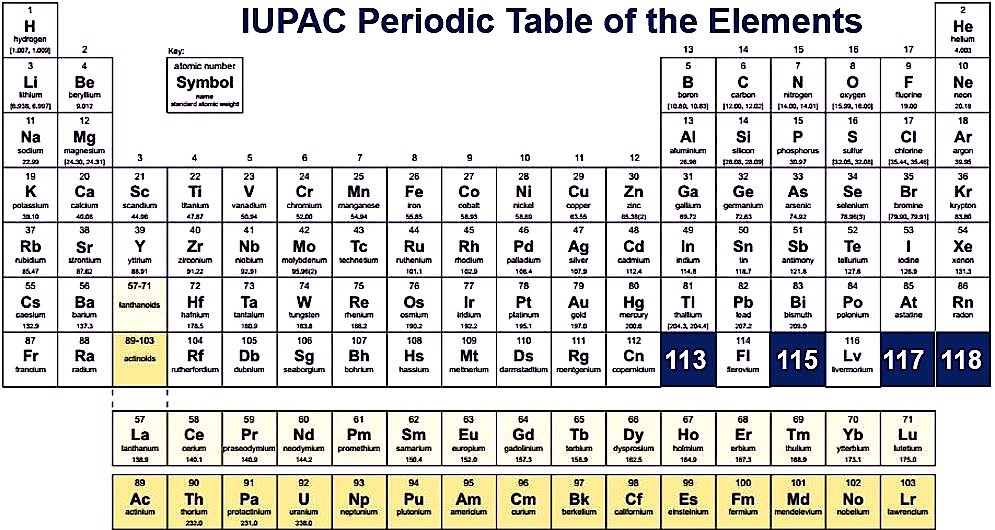
As we all know there are 118 elements in the periodic table and this periodic table cites keeping with most IUPAC values and by this, you can use it for your calculation and homework.
You can also print any interactive periodic table by your end which helps you to know more about this, there are some elements that are fundamentally different from the conventional periodic table and which are defined by charge positive or negative, and valence statements were taken by these elements and these are the few things which make the periodic table much more effective.
For this, there are also lots of books on the periodic table and every book provides the same information in different ways we can’t neglect this because with every book we learn something new.
There we see a basic table that students can also fill by their end which we call the Blank periodic table. A student can fill and this periodic table helps them improve learning.
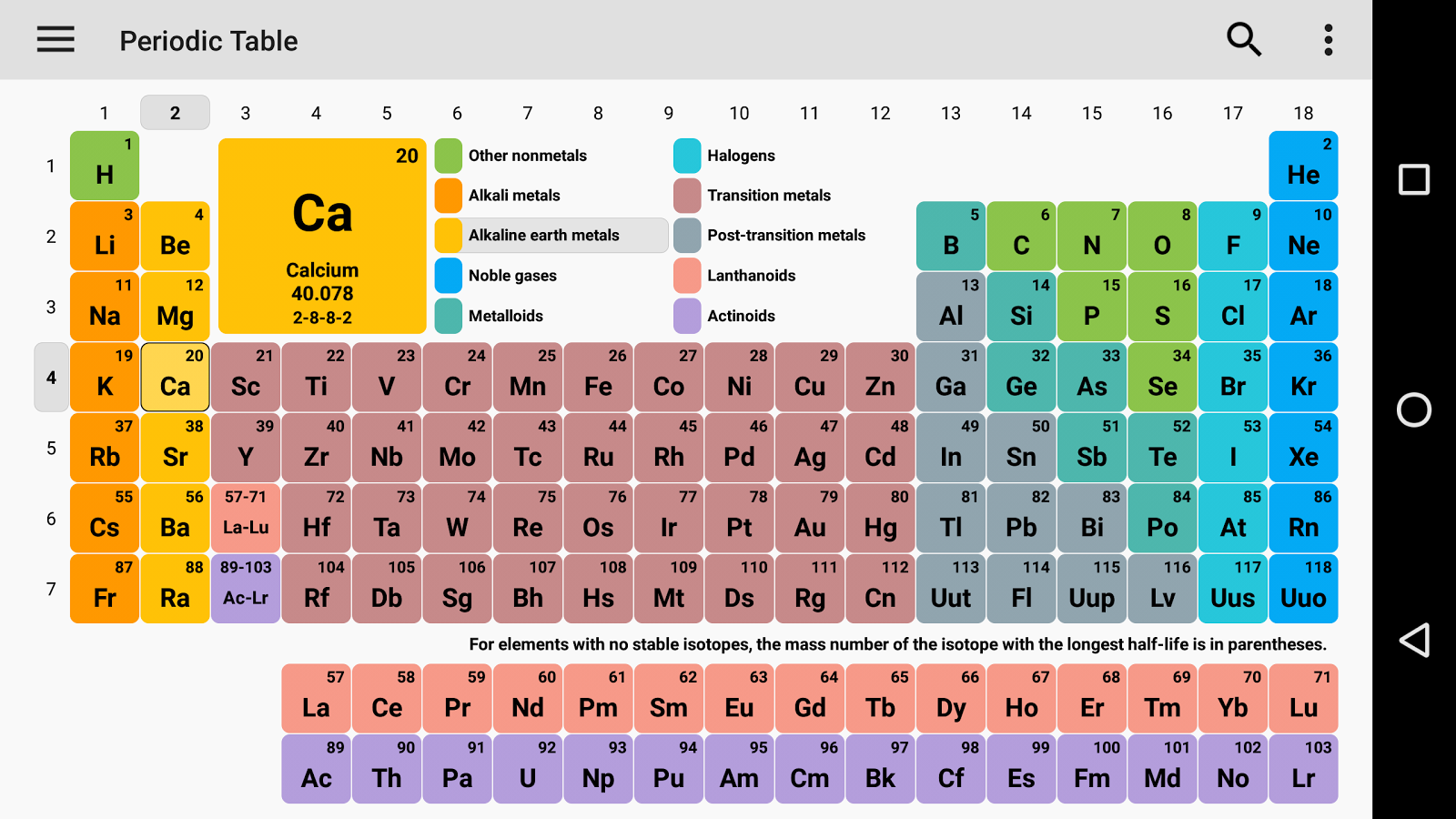
Periodic Table with Atomic Charges
As we all know the value of the periodic table is made by charges for atoms of the chemical elements. You can also use this periodic chart to know more about the charges of elements and one thing you know is how those elements make a bond with other elements. As we all know charges of an atom are based on the valence electron or oxidation state. The atom of elements is stable when its outermost cell is filled with outer electrons or half-filled. Those that have more electrons are considered stable atoms.
In the periodic table, Hydrogen is on top and it consists of 1 proton and 1 unpaired electron which we consider a free radical, and this unpaired electron is eagerly waiting for making a pair with other electrons.
When we listen to the word proton we easily assume that it means positive and it also means positive ions of hydrogen are known as a proton. Proton is a form of hydrogen that produces the ATP enzyme.
The hydride is hydrogen which has an extra electron so it takes a negative charge ion or anion, i.e. it takes a minus sign and it makes a regular Hydrogen atom.
Molecular hydrogen is the form where when two hydrogen atoms make a bond together and hydrogen molecules. H2 is also molecular hydrogen. It consists of two protons and two electrons, and it is the most common form of hydrogen because it is stable with neutral charges, it is also an antioxidant in hydrogen-rich water, molecular hydrogen is the smallest molecule in the universe, which means that no one can go this molecule can easily go.
Here you can also Download the Periodic Table with Charges of Elements.
Periodic Table of Elements with Names
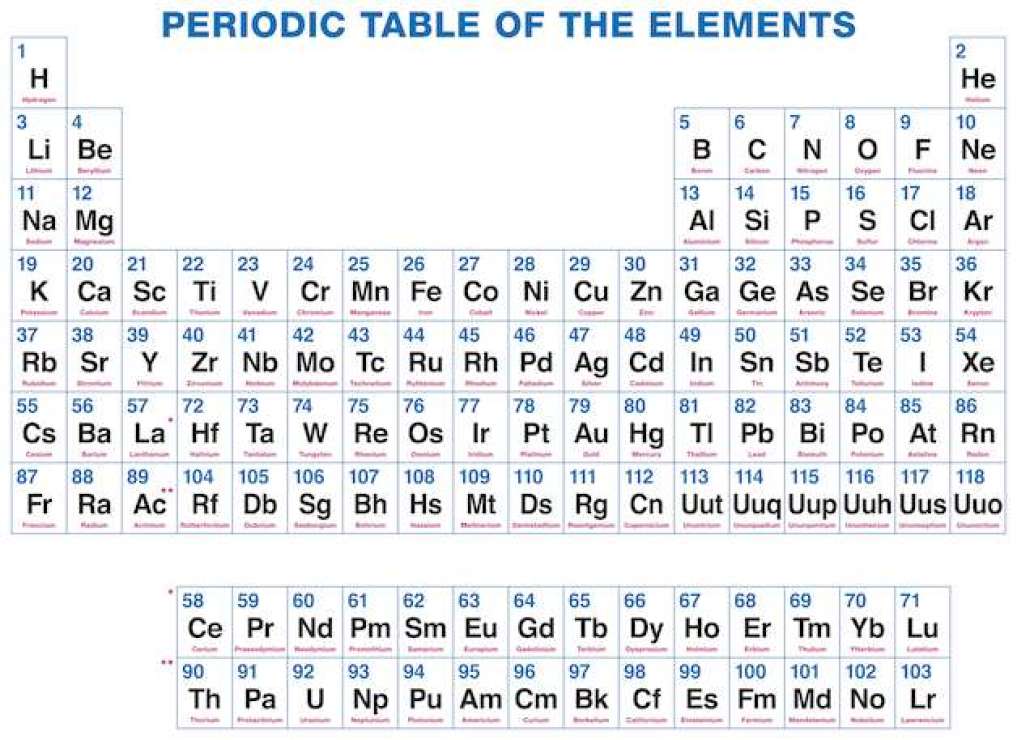
Now I provide you with all periodic table elements with names with the increasing form of an atomic number. As you know every element consists of one or two letters symbol, which is a kind of form of present and old names. The element’s number is its atomic number, which is the number of protons in atoms.
1 – A – Hydrogen
2 – He – helium
3 – Li – lithium
4 – Be – Beryllium
5 – B – Boron
6 – C – Carbon
7 – N – Nitrogen
8 – o – Oxygen
9 – F – Fluorine
10 – Do – Neon
11 – Na – Sodium
12 – Mg – Magnesium
13 – Al – aluminium
14 – Si – Silicon
15 – P – phosphorus
16 – S – Sulfur
17 – Cl – Chlorine
18 – Ar – Argon
19 – K – Potassium
20 – Ca – Calcium
21 – SC – Technetium
22 – Ti – Titanium
23 – 5 – Vanadium
24 – Cr – chromium
25 – MN – manganese
26 – Fe – Iron
27 – Co – Cobalt
28 – Ni – nickel
29 – Cu – Copper
30 – Zn – Zinc
31 – GA – Gallium
32 – Ge – Germanium
33 – As – Arsenic
34 – Se – Selenium
35 – br – Arsenic
36 – K – Krypton
37 – RB – Rubidium
38 – Sr – Strontium
39 – Y – yttrium
40 – Zr – Zirconium
41 – Nb – Bismuth
42 – Mo – molybdenum
43 – TC – technetium
44 – RU – ruthenium
45 – Rh – Rhodes
46 – Pd – Palladium
47 – Ag – Silver
48 – Cd – Cadmium
49 – IN – India
50 – Sn – Tin
51 – Sb – Antimony
52 – Te – Tellurium
53 – 1 – Iodine
54 – Xe – Xenon
55 – CS – caesium
56 – Ba – Barium
57 – La – lanthanum
58 – Ce – cerium
59 – Co- Cobalt
60 – Nd – neodymium
61 – PM – promethium
62 – Sm – samarium
63- Eu – Europium
64 – Gd – gadolinium
65 – Tb – terbium
66 – Dy – dysprosium
67 – Ho – holmium
68 – Er – erbium
69 – Tm – thulium
70 – Yb – ytterbium
71 – Lu – lutetium
72 – Hf – Hafnium
73 – Ta – Osmium
74 – W – Tungsten
75 – Re – rhenium
76 – OS – osmium
77 – Ir – Iridium
78 – Pt – Platinum
79 – Au – Gold
80 – HG – Mercury
81 – Tl – Thallium
82 – Pb – Lead
83 – Bi – bismuth
84 – Po – Bismuth
85 – but – astatine
86 – R – radon
87 – FR – France
88 – Ra – Radium
90 – Th – Thorium
91 – PA – Protactinium
92 – U – uranium to
93 – NP – Neptune
94 – pu – Plutonium
95 – Am – America
96 – Cm – Americium
97 – Bk – Berkelium
98 – CF – Kaliforniyum
99 – are – Einstein
100 – FM – Barium
101 – MD – Mendélévium
102 – No – Nobelium
103 – Lt. – Lawrence
104 – R – rutherfordium
105 – DB – Dubnium
106 – SG – Dubnium
107 – Hs – Bohrium
108 – Hs – Hass
109 – Mt – Meitnerium
110 – D – Darmstadtium
111 – RG – rubidium
112 – Cn – Copernicus
113 – NH – Nihon
114 – Fl – Flerov
115 – Mc – Moscow
116 – LV – Livermorium
117 – TS – Tennessee
118 – og – Oganesson
Dynamic Periodic Table
By dynamic periodic table, you get all information about the periodic table, and here can easily cover all information regarding every element including everything like electrons, protons, neutrons, electrons on outermost orbits and temperature, etc. and you can visualize the data of the periodic table, and here you can know easily about every element which takes place in the periodic table and I must suggest you go through it because here I tell you about all atoms which one is positive which one is negative and which one is neutral, by some images, you get all information, Dynamic periodic table means were you get all information by images and it is the tabular arrangement of chemical elements, and which is ordered by their atomic number.
All elements from hydrogen to oganesson have been discovered or synthesized. The first 94 elements are natural elements and the rest of the elements are tested in laboratories or nuclear reactors.
There are also some changes in the periodic table and new 4 elements are taken Nihonium, Moscovium, Tennessee, and Oganesson. This was confirmed by (IUPAC) on December 30, 2015, and officially named on November 28, 2016. And the atomic number recently added 113,115,117 and 118. This method tries to cover all methods from school to an expert in the industry. In this way, I can try to tell everyone about the periodic table.
Blank Periodic Table
Compared to another periodic table, the Blank periodic table is best because if I see in this we fill the periodic table by your end and for learning you can use this if you know all things about the periodic table and then fill all columns and easily test your knowledge, you can also use this for the practice of periodic table and also use this on your textbook.
For this, you need to just download the periodic table PDF on a device and you can also take the print on A4 sheet paper. In this blank column, you can fill in all atomic numbers, periods, and groups if desired. You can also fill optional color in a different column like alkali metals, transaction metals, lanthanides, actinides, metalloids, halogens, and noble gases. And the best part is if you make a mistake then you can easily improve that mistake because there you have also a chance to do that and as I already told you can also take as many as a page of blank printable periodic, by this student can also prepare for the test, and also test their knowledge.
You Can Download Here Blank Periodic Table Chart
Periodic Table of Elements Printable
In this you can easily find a perfect printable table for your needs, here you can get a complete collection of a free printable periodic table in PDF files or PNG images to save, print, and use. We are trying to put complete information about the periodic table and all HD images where you put as a screen saver on your devices.
You can fit it as you can on your device, and you can easily fit on your device, and when you take print at that time also you can fit it as you can on a sheet of paper, and by taking a print you can also use it for your personal use like you can take this page for lab calculations, this table re-sized, so you can view or print at any size from small to large poster size according to your choice. In this, you can easily get all 118 elements in fact and figures. In this printable periodic table the atomic masses and the significant figures and keeping with the most recent IUPAC values.
IUPAC Values
As I already told you, you can use it for your test and by this, you can easily test your knowledge and in this, there are not any boundaries, you can take print as much as you can, and the best part is there is several images which is in HD and this you can also make your wallpaper on your device like cell phone, laptop, tab, and many more things and by this, you always remember about these things.
To remember this thing you can also read books as much as you can because by this you know only about an atomic numbers, atomic mass, and many more things, but after reading books you can also know about how you can use this practice, because as we all know science is only depend on proof they never depend on other things so my suggestion is very simply put as much effort as you can by which you can improve your learning’s and also get result regarding your learning’s.
If you Can Search For More Details Go Here: Periodic Table of Elements
Printable Table Trends
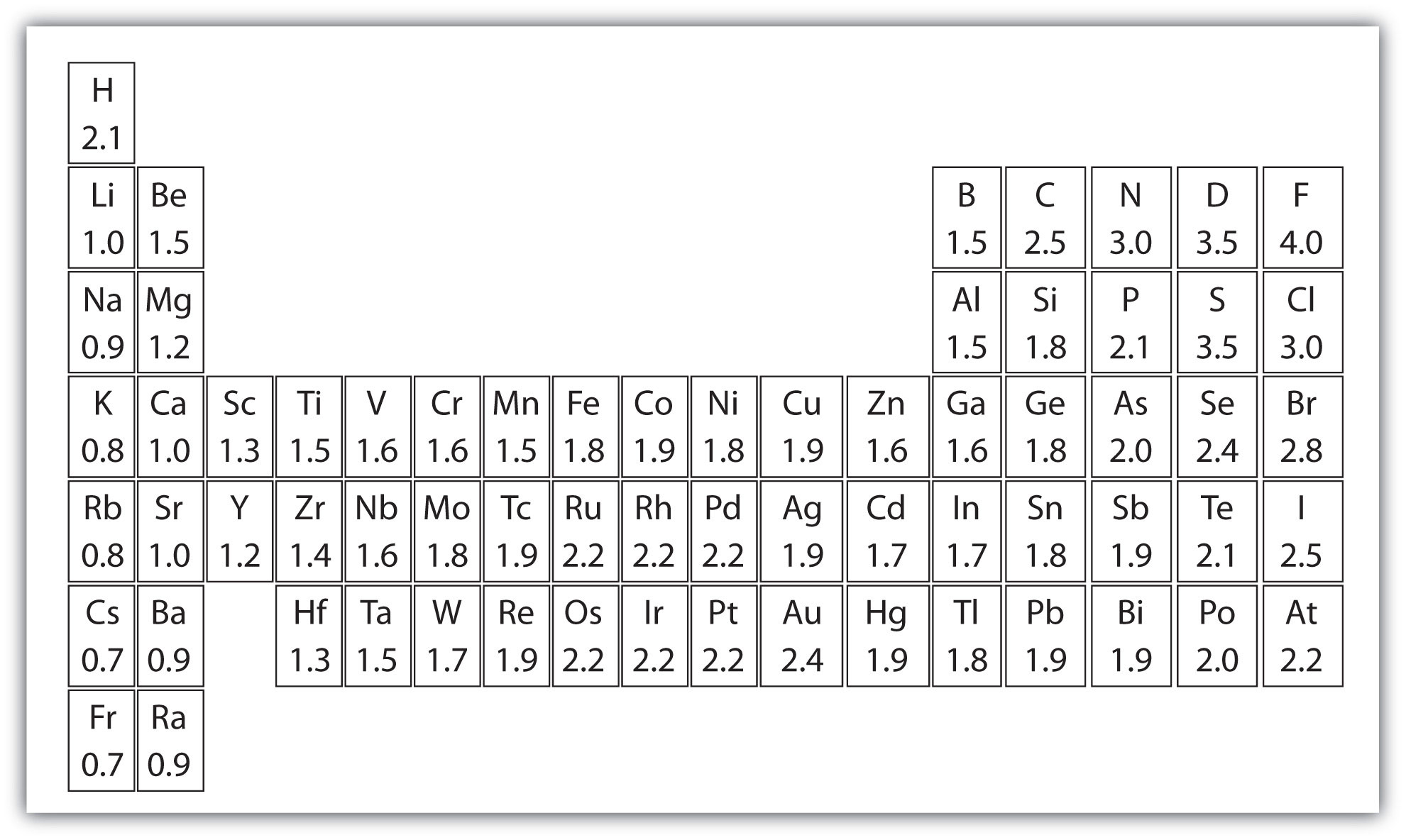
You can use this chart to see the beauty of the periodic table in terms of electronegativity, ionization energy, atomic radius, metallic character, and electron affinity. These elements structurally fit on a periodic table. If I talk about electronegativity it shows how easily chemicals form a chemical bond, and as we see it is an increase from left to right and decrease a from right to left in a group. We noticed that noble gasses (that column which is on the right-hand side) are relatively inert so i.e. electronegativity approaches zero (we can’t take its overall trend).
Any atom has a large difference in electronegativity values, more atoms form a bond which is known as a chemical bond.
Periodic table trends chart
Here you can Go Through the Periodic Table Trends
Ionization energy is considered the smallest amount of energy and needs help an electron away from an atom in the form gaseous state. It increases the energy of ionization when you move from left to right because it increases the number of protons more strongly and it is really hard to remove that.
Nuclear sweep is the separation from the core to the peripheral stable electron while the ionic range is a large portion of the separation between two nuclear cores that are simply touching each other. This related esteem show a similar pattern in the occasional table.
As you move down the occasional table, components have more protons and pick up an electron vitality shell, so iotas wind up noticeably bigger. As you move over a column of the intermittent table, there are some more protons and electrons in it, yet the electrons are held all the more intently to the core, so the general size of the iota diminishes.
Many elements in the periodic table are metals, it means they show a metallic character. All Properties of metals like metallic lustre, high electrical and thermal conductivity, ductility, malleability, and several other traits. Periodic table right-hand side contains the nonmetals, which do not shows all these properties. As with many other properties, the metallic character which relates to the configuration of valence electrons.
The vast majority of the components in the intermittent table are metals, which implies they show metallic character. Properties of metals incorporate metallic brilliance, high electrical and warm conductivity, pliability, flexibility, and a few different qualities. The right-hand side of the occasional table contains the nonmetals, which don’t show these properties. Likewise, with alternate properties, metallic character identifies with the arrangement of valence electrons.
Printable Table Wallpaper
There you get lots of printable wallpaper in which you get complete information about the periodic table as you get by another way, in this also you know about the element name, element family, atomic number, atomic weight, element symbol, group, and atomic mass. In this image, you see all the information and it seems a very light image so you don’t face any kind of problem seeing it and it also resizes cleanly for other resolutions. This wallpaper of the periodic table fresh update of the original chart featured on this site.
There you also get some transparent wallpaper in HD quality, by this you don’t need to face any problems; easily you see images without any pressure or effect on the eyes, And this image also you can download and make your wallpaper. And this really helps you to make your notes and projects.
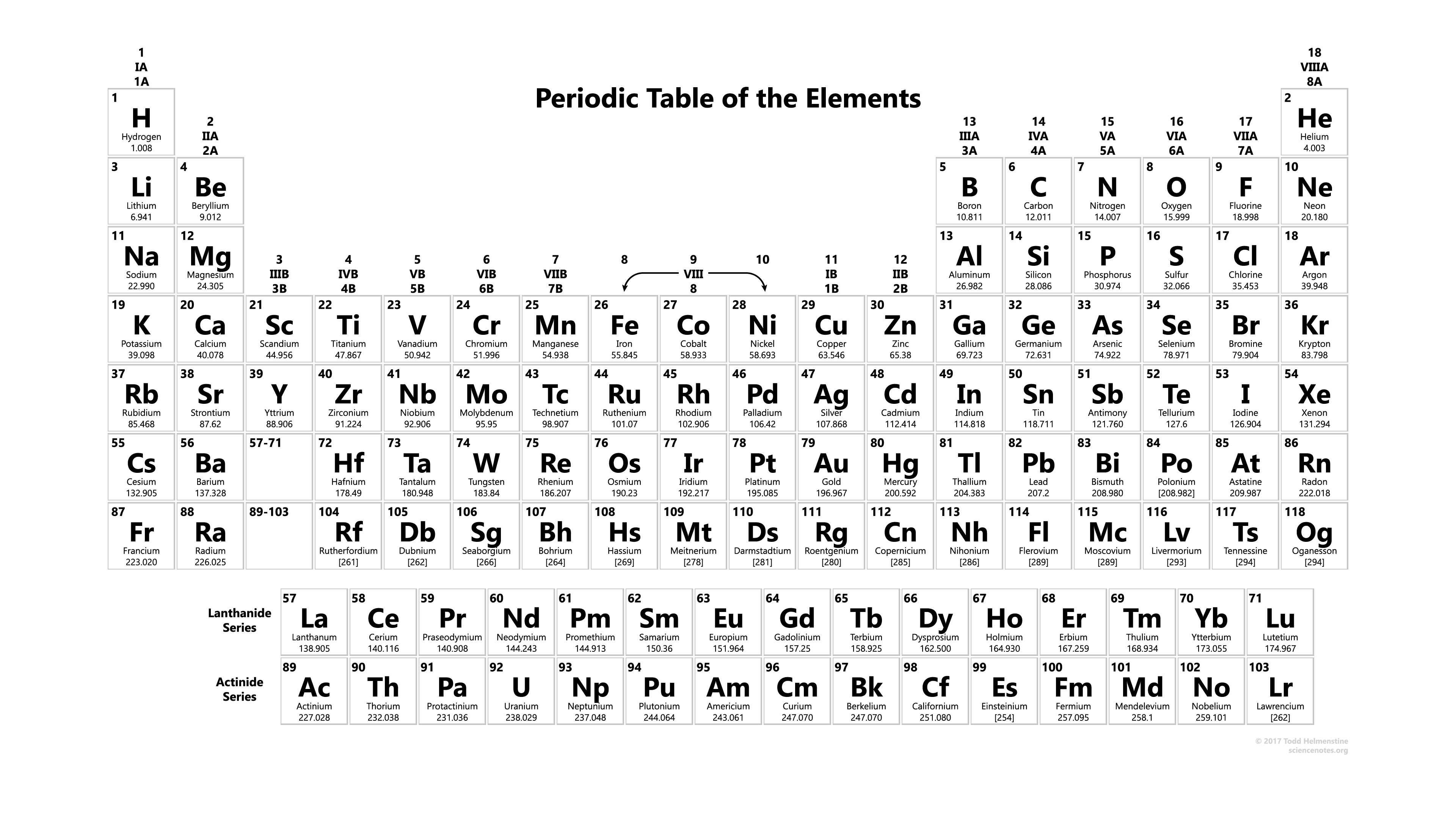
Printable Periodic Table With Atomic mass
| Atomic No. | Symbol | Element Name |
Atomic Mass (u)
|
| 1 | H | Hydrogen | 1.008 |
| 2 | He | Helium | 4.0026 |
| 3 | Li | Lithium | 6.94 |
| 4 | Be | Beryllium | 9.0122 |
| 5 | B | Boron | 10.81 |
| 6 | C | Carbon | 12.011 |
| 7 | N | Nitrogen | 14.007 |
| 8 | O | Oxygen | 15.999 |
| 9 | F | Fluorine | 18.998 |
| 10 | Ne | Neon | 20.18 |
| 11 | Na | Sodium | 22.99 |
| 12 | Mg | Magnesium | 24.305 |
| 13 | Al | Aluminum | 26.982 |
| 14 | Si | Silicon | 28.085 |
| 15 | P | Phosphorus | 30.974 |
| 16 | S | Sulfur | 32.06 |
| 17 | Cl | Chlorine | 35.45 |
| 18 | Ar | Argon | 39.948 |
| 19 | K | Potassium | 39.098 |
| 20 | Ca | Calcium | 40.078 |
| 21 | Sc | Scandium | 44.956 |
| 22 | Ti | Titanium | 47.867 |
| 23 | V | Vanadium | 50.942 |
| 24 | Cr | Chromium | 51.996 |
| 25 | Mn | Manganese | 54.938 |
| 26 | Fe | Iron | 55.845 |
| 27 | Co | Cobalt | 58.933 |
| 28 | Ni | Nickel | 58.693 |
| 29 | Cu | Copper | 63.546 |
| 30 | Zn | Zinc | 65.38 |
| 31 | Ga | Gallium | 69.723 |
| 32 | Ge | Germanium | 72.63 |
| 33 | As | Arsenic | 74.922 |
| 34 | Se | Selenium | 78.971 |
| 35 | Br | Bromine | 79.904 |
| 36 | Kr | Krypton | 83.798 |
| 37 | Rb | Rubidium | 85.468 |
| 38 | Sr | Strontium | 87.62 |
| 39 | Y | Yttrium | 88.906 |
| 40 | Zr | Zirconium | 91.224 |
| 41 | Nb | Niobium | 92.906 |
| 42 | Mo | Molybdenum | 95.95 |
| 43 | Tc | Technetium | -98 |
| 44 | Ru | Ruthenium | 101.07 |
| 45 | Rh | Rhodium | 102.91 |
| 46 | Pd | Palladium | 106.42 |
| 47 | Ag | Silver | 107.87 |
| 48 | Cd | Cadmium | 112.41 |
| 49 | In | Indium | 114.82 |
| 50 | Sn | Tin | 118.71 |
| 51 | Sb | Antimony | 121.76 |
| 52 | Te | Tellurium | 127.6 |
| 53 | I | Iodine | 126.9 |
| 54 | Xe | Xenon | 131.29 |
| 55 | Cs | Cesium | 132.91 |
| 56 | Ba | Barium | 137.33 |
| 57-71 | Lanthanides | ||
| 72 | Hf | Hafnium | 178.49 |
| 73 | Ta | Tantalum | 180.95 |
| 74 | W | Tungsten | 183.84 |
| 75 | Re | Rhenium | 186.21 |
| 76 | Os | Osmium | 190.23 |
| 77 | Ir | Iridium | 192.22 |
| 78 | Pt | Platinum | 195.08 |
| 79 | Au | Gold | 196.97 |
| 80 | Hg | Mercury | 200.59 |
| 81 | Tl | Thallium | 204.38 |
| 82 | Pb | Lead | 207.2 |
| 83 | Bi | Bismuth | 208.98 |
| 84 | Po | Polonium | -209 |
| 85 | At | Astatine | -210 |
| 86 | Rn | Radon | -222 |
| 87 | Fr | Francium | -223 |
| 88 | Ra | Radium | -226 |
| 89-103 | Actinides | ||
| 104 | Rf | Rutherfordium | -267 |
| 105 | Db | Dubnium | -270 |
| 106 | Sg | Seaborgium | -271 |
| 107 | Bh | Bohrium | -270 |
| 108 | Hs | Hassium | -277 |
| 109 | Mt | Meitnerium | -278 |
| 110 | Ds | Darmstadtium | -281 |
| 111 | Rg | Roentgenium | -282 |
| 112 | Cn | Copernicium | -285 |
| 113 | Nh | Nihonium | -286 |
| 114 | Fl | Flerovium | -289 |
| 115 | Mc | Moscovium | -290 |
| 116 | Lv | Livermorium | -293 |
| 117 | Ts | Tennessine | -294 |
| 118 | Og | Oganesson | -294 |
In this, you get complete information about the atomic mass of all elements which you use when you are doing studies or when you are doing some experiments in a lab, and you can also use this or you can also take the print for use. You get also information about the atomic masses of elements and in this, you also know further information on chemical properties, environmental data, or health effects, this list contains about 118 elements of chemistry and by this, you know about every element’s atomic mass.
Download From Here: Periodic Table With Atomic Mass
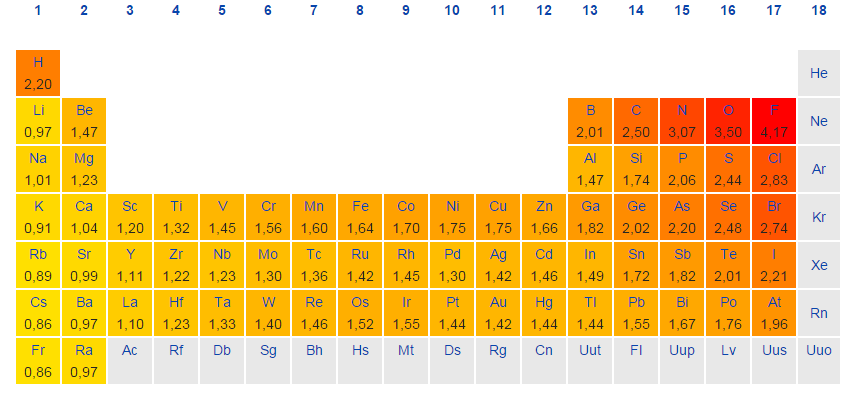
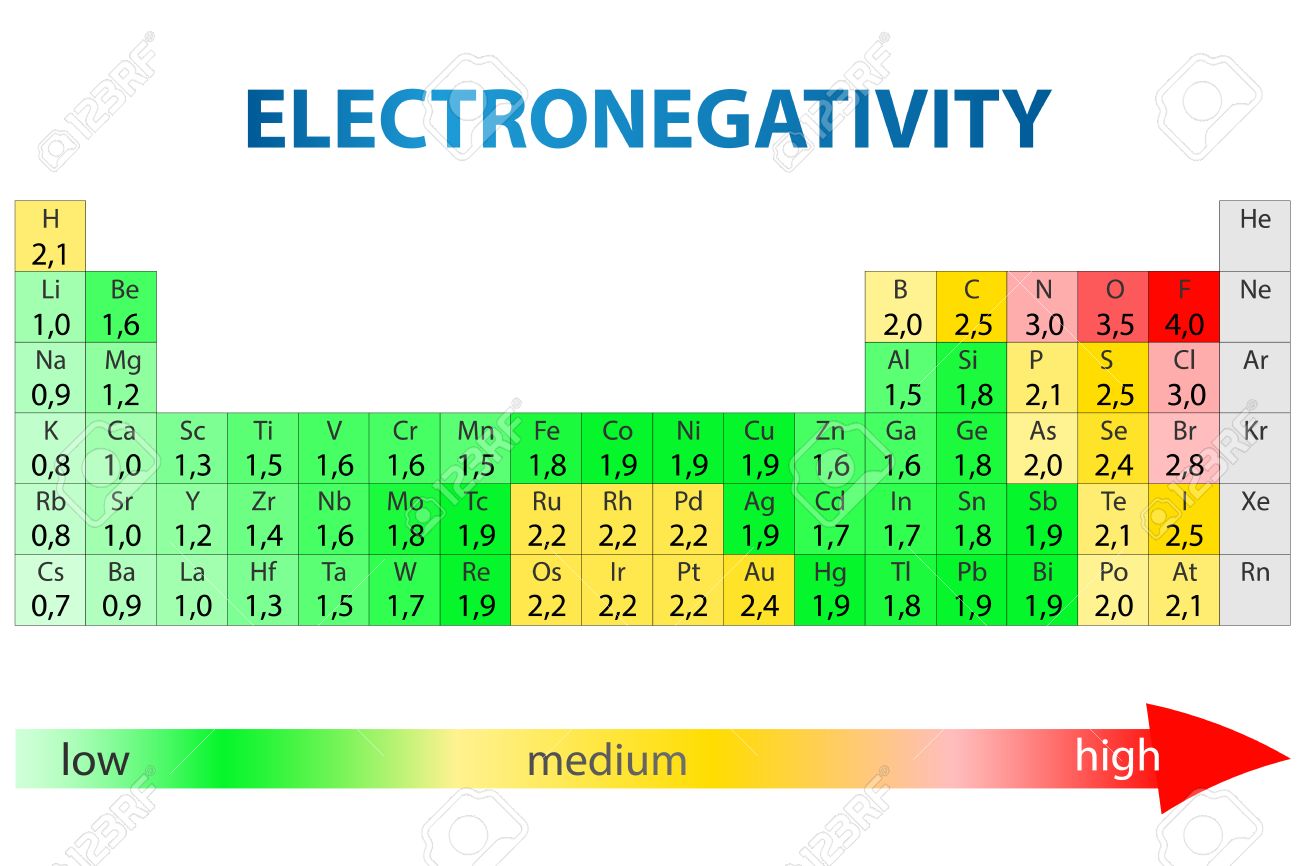
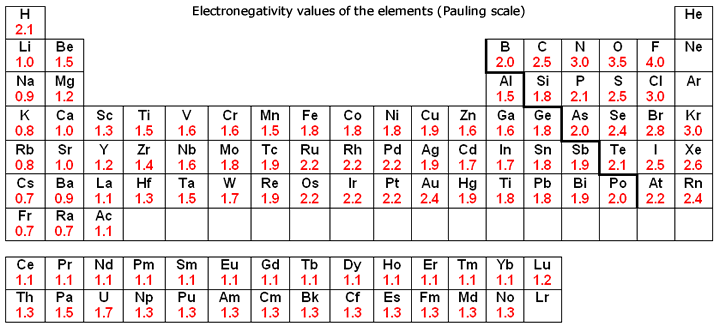
Periodic Table With Electronegativity
Electronegativity alludes to the capacity of an iota to pull in shared electrons in a covalent bond. The higher the estimation of the electronegativity, the all the more unequivocally that component pulls in the mutual electrons.
Black and White Periodic Table
The idea of electronegativity was presented by Linus Pauling in 1932; on the Pauling scale, fluorine is doled out an electronegativity of 3.98, and alternate components are scaled with respect to that esteem. Other electronegativity scales incorporate the Mulliken scale, proposed by Robert S. Mulliken in 1934, in which the principal ionization vitality and electron partiality are found in the middle value together, and the Allred-Rochow scale, which measures the electrostatic fascination between the core of an iota and its valence electrons.
Electronegativity changes typically over the intermittent table. Electronegativity increments from base to top in gatherings, and increments from left to appropriate crosswise over periods. Therefore, fluorine is the most electronegative component, while francium is one of the slightest electronegative. (Helium, neon, and argon are not recorded in the Pauling electronegativity scale, despite the fact that in the Allred-Rochow scale, helium has the most noteworthy electronegativity.)
Interactive Periodic Table
An interactive periodic table is all about how much you know the periodic table, and how much you are involved with this, if you see there is just a number and an alphabet but if you understand you see a complete world in this which is related to science and this really help in all terms.
As we know atoms are really important in this which is composed of subatomic particles like protons, neutrons, and electrons. These particles have different- different arrangements with giving electrons, the thing that we have to remember in every condition is that proton, neutron, and electron are exactly the same as the proton, neutron, and electron of different other elements, basically, it is arrangements of the number that makes the different elements. But before that, we generally ask a question what is proton, neutron, or electron? so here I am trying to give an answer.
Interactive periodic table for kids
A proton is a positively charged particle, weighing 1 atomic mass unit (1.67xgrams) and this one is located in the nucleus. Neutrons are neutrally negative charged particles and weigh approximately 1 atomic mass and this one is located in the nucleus.
Electrons are negatively charged particles and it is weighing as zero atomic mass units it is located in the various orbital of the energy level and the weight of electrons is 9.11x which means it takes 1830 electrons to equal the mass of one proton.
The occasional table of the substance components is a rundown of known Elements. In the table, the components are submitted at the request of their nuclear numbers beginning with the least number. The nuclear number of a component is the same as the number of protons in that specific atom. In the intermittent table, the components are masterminded into periods and gatherings. A column of components over the table is known as a period. Every period has a number: from 1 to 8. Period 1 has just 2 components in it: hydrogen and helium. Period 2 and Period 3 both have 8 components. Different periods are longer. Components in a period have continuous nuclear numbers.
Free Periodic PDF Chart
A segment of components down the table is known as a gathering. There are 18 bunches in the standard occasional table. Each gathering has a number: from 1 to 18. Components in a gathering have electrons masterminded in comparable ways, which gives them comparable concoction properties (they carry on in comparative ways). For instance, assemble 18 is known as the honorable gases since they are for the most part gases and they don’t consolidate with different molecules.
There are two frameworks for gathering numbers; one utilizing Arabic numerals (1,2,3) and the other two utilizing Roman numerals (I, II, III).
The Roman numeral names were utilized at first and are the customary names; the Arabic numeral names are more up to date names that the International Union of Pure and Applied Chemistry (IUPAC) chose to use also. The IUPAC names were intended to supplant the more established Roman numeral frameworks. The Periodic Table was imagined and orchestrated by the Russian physicist Dmitry Ivanovich Mendeleyev (1834-1907). In his respect, component 101 was named after him, mendelevium.
Here are the Periodic Table PDF Elements
The periodic table is also used by chemists to observe the pattern and relationship between the elements that which element is related to which element also there are 3 main groups in the periodic table metal, metalloids, and gases.
Periodic Table with Mass
As we all know every element has its unique mass, which is measured by a number of experts in a laboratory, and then the result comes out and mass weight is very important because by this we get the result and we get how element we use for the particular experiment.
Mass is important in many terms and by the periodic table we know easily which element has how much weight, so for this, you need to just download a periodic table with a mass that by you know how much any contains. And you can also carry with you a single sheet of paper.
P Table Orbitals
As we all know about the periodic table every cell is divided by its orbitals and it is a kind of region of probability where the electron can be found. And these entire regions have their shape.
There are several orbitals like S orbitals, P orbitals, and D orbitals, and every orbital has its uniqueness and every orbital has its block also.
S, P, D, F Orbitals Periodic Table
Like if I talk about S orbitals is the first place of an electron and it is located in an atom. Then P orbital has its uniqueness in that it has been filled with full of energy level, electrons start filling in the next higher orbit style. D orbitals also have their uniqueness and there are two other styles which is a little complex to understand.
At last, I try to explore my all knowledge about the periodic table and if you get what you want from this site, then I suggest you share with friends also that this site they can also explore their knowledge, and if you have some suggestions for us then please comment below.