Oxygen Electron Configuration: O is an odourless, colorless, reactive gas. Its atomic number is 8 and it the life-supporting component of the air. It forms almost 20 percent of the earth’s atmosphere. It is also the most abundant element in the earth’s crust which is found mainly in the form of silicates, oxides, and carbonates.
- H Electron Configuration
- He Electron Configuration
- Li Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- C Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Vanadium Electron Configuration
- Clorine Electron Configuration
Oxygen Electron Configuration
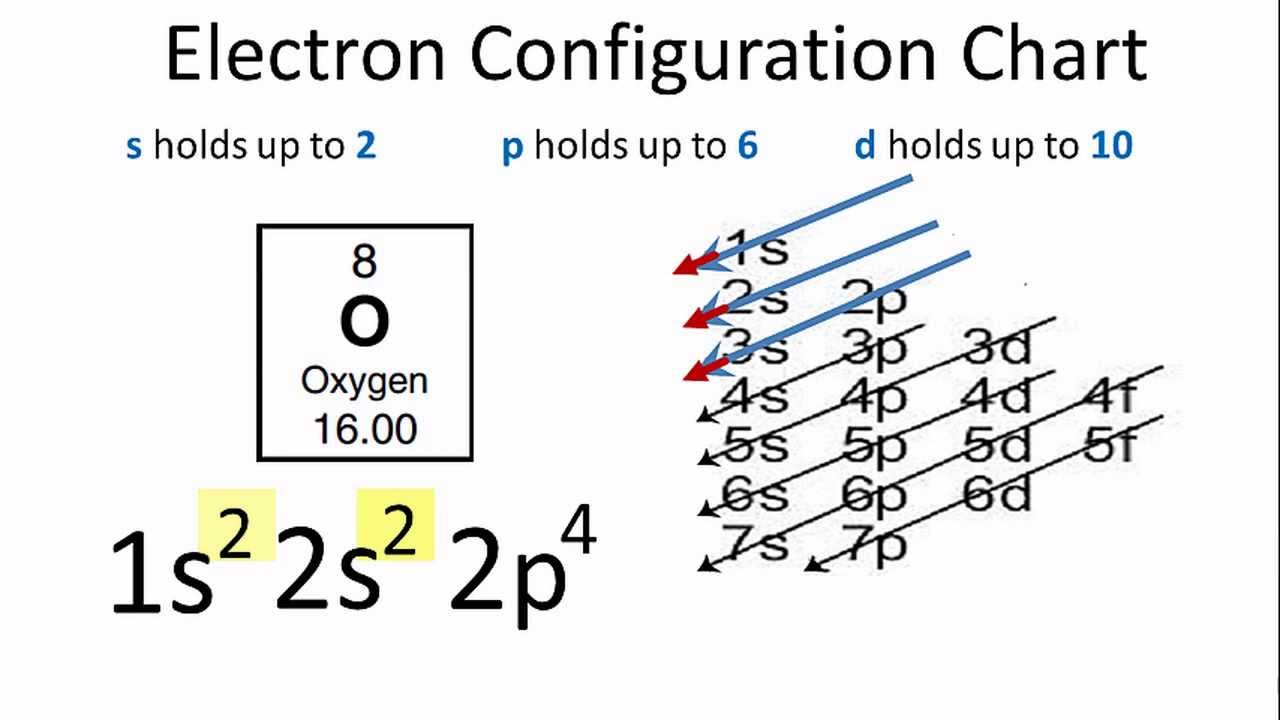
The symbol for Oxygen is O. It is one of the members of the chalcogen group in the periodic table and also a highly reactive nonmetal. It an oxidizing agent which instantly forms oxides with most of the elements and other compounds. Today we are here to give you the information of O and its electron configuration.
Electron Configuration For O Ion
The electron configuration for O Ion is [He] 2s2 2p4.
Full Electron Configuration For Oxygen
Oxygen’s Atomic no. = 8 So the configuration is 1s22s22p4
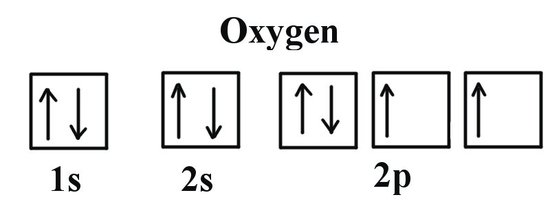
Ground State Electron Configuration For Oxygen
See the image below for ground state electron configuration of O.
What is the Electron Configuration of O
The distribution of electrons of an atom or molecule in atomic or molecular orbitals is called the electron configuration. For example, the electron configuration of the oxygen is [He] 2s2 2p4. Oxygen is very important element of periodic table and many other gases and liquid can be created with the combination of O.
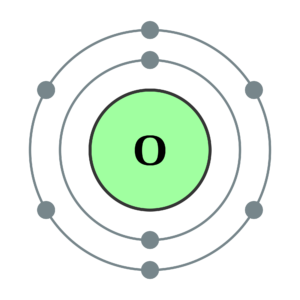
How many Valence Electrons are in Oxygen
There is Six valence electron in the Oxygen. You can see the above Orbital Dot Diagram. The symbol of Oxygen is O and the atomic Mass of Oxygen is 16.


Leave a Reply