Hydrogen Electron Configuration: Electron configuration can be defined as the number of electrons present in the atom or molecule’s orbit. Hydrogen is the chemical element that is the first element of the periodic table and has atomic number one (1). Its symbol is “H”. It is the lightest element of the periodic table and monatomic element as well.
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
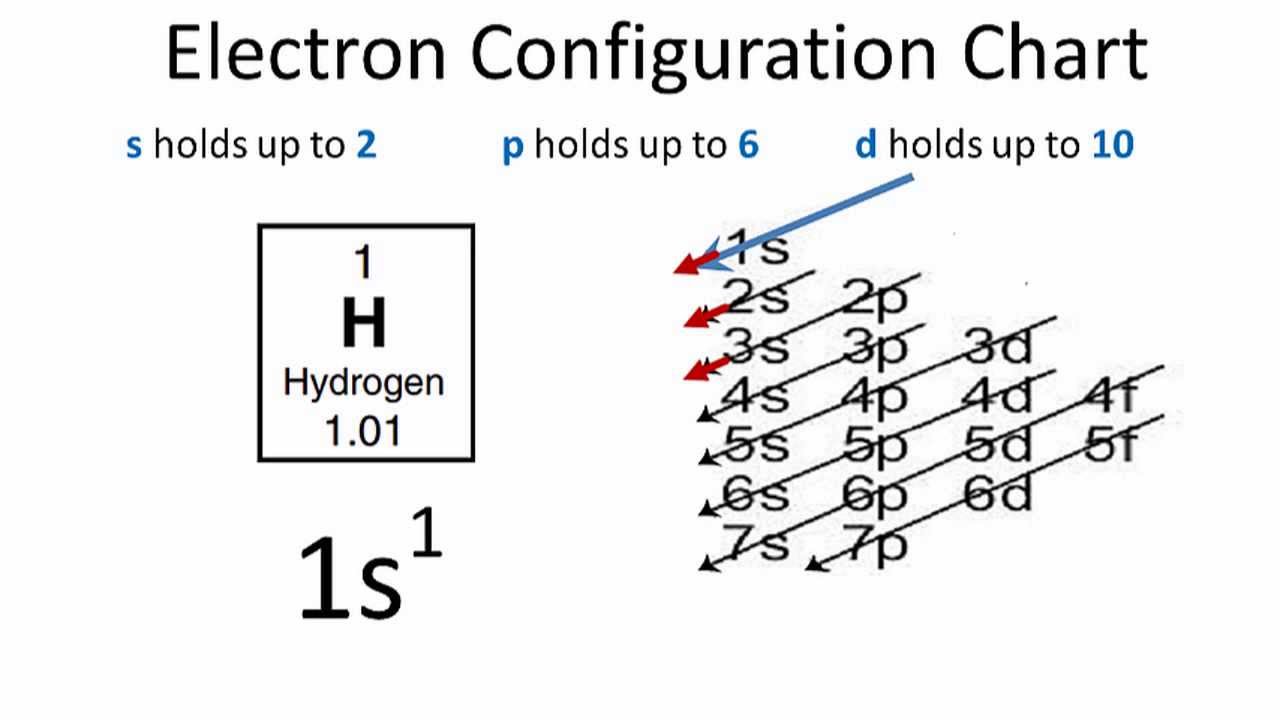
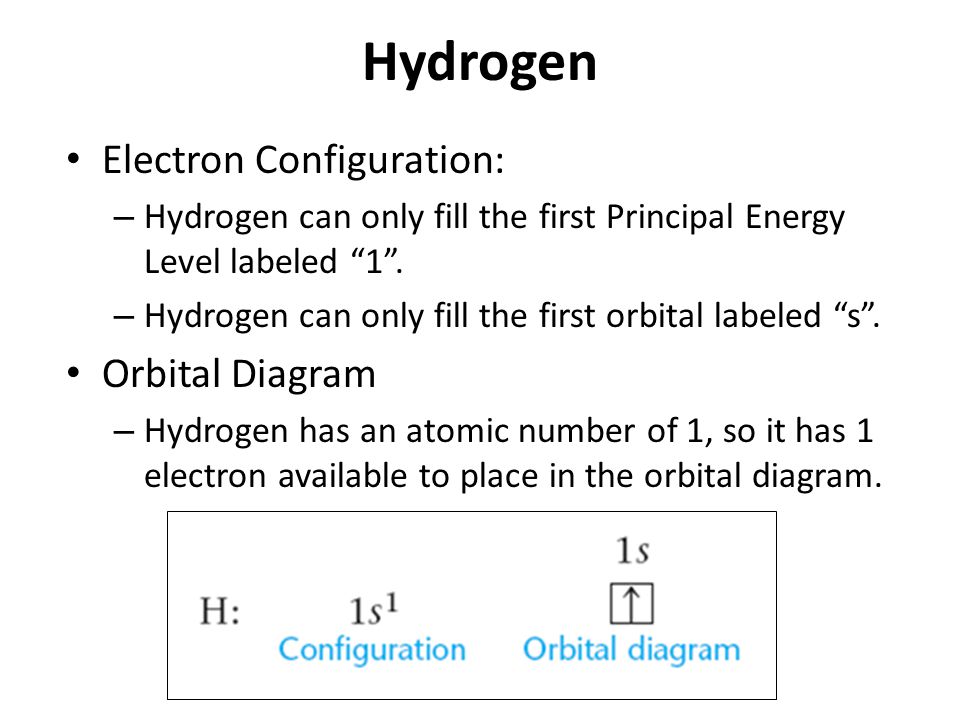
Electronic configuration of Hydrogen can be written as 1s1.
As in the case of the Hydrogen element, there is only one electron which is present in one shell.
Hydrogen Electron Configuration
Electrons distribution in the atom’s orbit is termed as electron configuration. Hydrogen as the monatomic element has only one electron and its full electron configuration can be defined as 1s1. Its one electron is present in the 1s orbit of the Hydrogen atom.
What is The Electron Configuration of H2
Electron configuration is the number of electrons that are present in the orbit of atom or molecules. The electron configuration of H2 is 1s1.
How Many Valence Electrons are in Hydrogen
The valence electrons are those electrons that are present in the outermost shell of the atom. But in the case of hydrogen, there is only one electron that is present in its first shell. So, the valence electron of hydrogen is also one. Or The number of valence electrons in Hydrogen is 1 (one).
Leave a Reply