Today we shall take up the topic of Hydrogen Valence Electrons. Valency is an important concept in chemistry and has been used to formulate many theories in chemistry and science in general. It can be found by looking at the group number of an element in a periodic table.
- Flerovium Valence Electrons
- Mercury Valence electrons
- Moscovium Valence Electrons
- Bismuth Valence electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Oganesson Valence Electrons
- Tellurium Valence Electrons
- Nobelium Valence Electrons
- Xenon Valence Electrons
- Neptunium Valence Electrons
- Cesium valence electrons
- Plutonium Valence Electrons
- Iodine Valence Electrons
- Americium Valence Electrons
- Radium Valence Electrons
- Gold Valence electrons
- Lead Valence electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Oxygen Valence Electrons
- Fluorine Valence Electrons
- Neon Valence Electrons
- sodium Valence Electrons
- Magnesium Valence Electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Phosphorus Valence Electrons
- Sulfur Valence Electrons
- Chlorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Calcium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
How Do You Find the Valency of Hydrogen
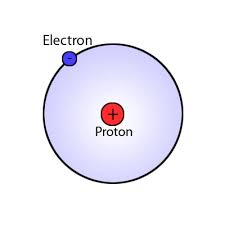
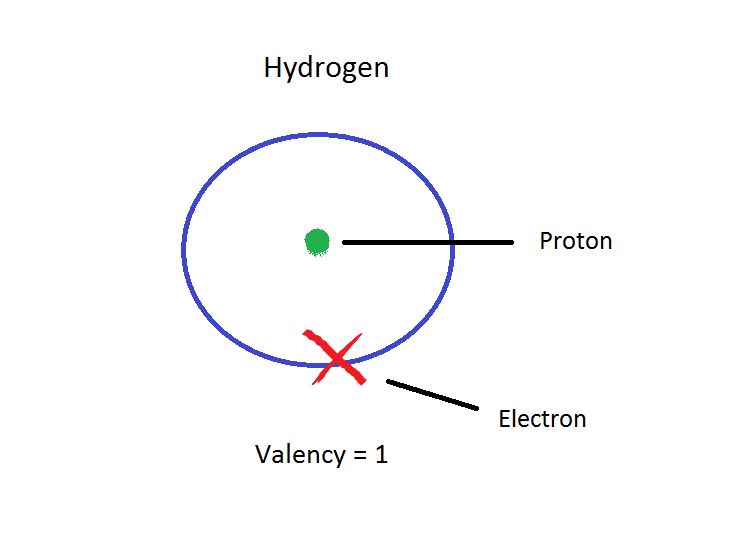
Since Hydrogen has only one electron in its shell, its valency is 1. It can also find out by looking at the periodic table since Hydrogen belongs to group 1. So to achieve stability it needs to share a bond with only one electron of another atom.
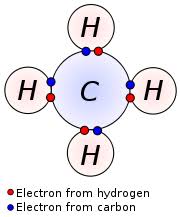
Hence one hydrogen atom can form bonds with electrons of many atoms simultaneously. For example, four Hydrogen atoms can form a bond with a carbon atom, to form the compound methane (CH4).
Hydrogen Valence Electrons
Since Hydrogen belongs to the first group of elements its electronic configuration is 1. Hence it belongs to the first group of the periodic table. And if the electronic configuration of any element is less than or equal to four, its valency can be determined by the group it belongs to in the periodic table.
How many Valence electrons does Hydrogen have?
A Hydrogen atom has only one electron in its outermost shell. Hence it requires combining with one more electron of an atom to achieve stability. So its number of valence electrons is 1.
Leave a Reply