Today we shall discuss the valency of another important element, i.e., iron valence electrons. As you know, valency is the measure of the capacity of a particular element to react and combine or displace the electrons of atoms of another element.
- Flerovium Valence Electrons
- Plutonium Valence Electrons
- Mercury Valence electrons
- Neptunium Valence Electrons
- Moscovium Valence Electrons
- Cesium valence electrons
- Bismuth Valence electrons
- Silicon Valence Electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Xenon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Hydrogen Valence Electrons
- Phosphorus Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Sodium Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Aluminum Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Fluorine Valence Electrons
- Magnesium Valence Electrons
- Neon Valence Electrons
- Sulfur Valence Electrons
If the number of electrons in an atom is less than or equal to four, the number of valence electrons is equal to the number of electrons in its outer shell. If it is more than four, it is eight minus the number of electrons in the outer shell.
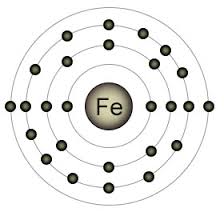
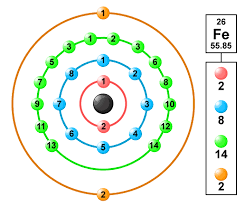
Iron Valence Electrons
Iron has an atomic number of 26. The valence electrons can vary from -2 to +6, but the most common valency it exhibits is +2 and +3.
Iron Valency & Electron Configuration

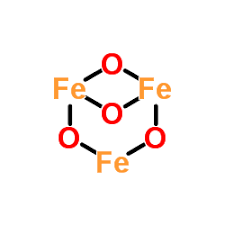
What is the Valency of Iron in Fe3O4
How many valence electrons does iron have?
Oxide ions tend to have a 2- charge. The overall charge of Fe3O4 is 3(Fe) + 4(-2) = 0, which means that the valency of iron in Fe3O4 is +8/3. But since having fractional valencies is not possible (you can only lose a whole number of electrons, but not fractions of an electron), this is only an average valency for the iron atoms in Fe3O4.
Leave a Reply