Hello friends! An important part of learning about the periodic tables is to know about Periodic Table Family Names. As you know, a periodic table has groups as well as periods. The group contains elements which have the same number of valence electrons. Since the elements in a group show similar chemical properties, they belong to a particular family.
- Hydrogen Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Oxygen Valence Electrons
- Fluorine Valence Electrons
- Neon Valence Electrons
- Sodium Valence Electrons
- Magnesium Valence Electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Phosphorus Valence Electrons
- Sulfur Valence Electrons
- Chlorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Iron Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
- Germanium Valence Electrons
- Arsenic Valence Electrons
- Selenium Valence Electrons
- Bromine Valence Electrons
- Krypton Valence Electrons
- Rubidium Valence Electrons
- Strontium Valence Electrons
- YttriumValence Electrons
- Zirconium Valence Electrons
- Niobium Valence Electrons
- Molybdenum Valence Electrons
- Technetium Valence Electrons
- Ruthenium Valence Electrons
- Rhodium Valence Electrons
- Palladium Valence Electrons
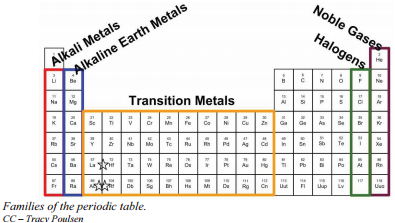
For example, the first group belongs to the lithium family and belong to the alkali metal category.
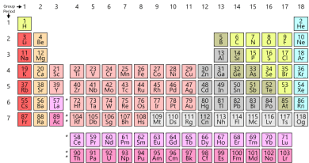
Periodic Table With Groups And Families
Groups are the columns across the periodic table. The elements belonging in the same group have same the valence electrons. These elements also have similar chemical properties.
For example, lithium family, beryllium family, nickel family are some of the families in a periodic table.
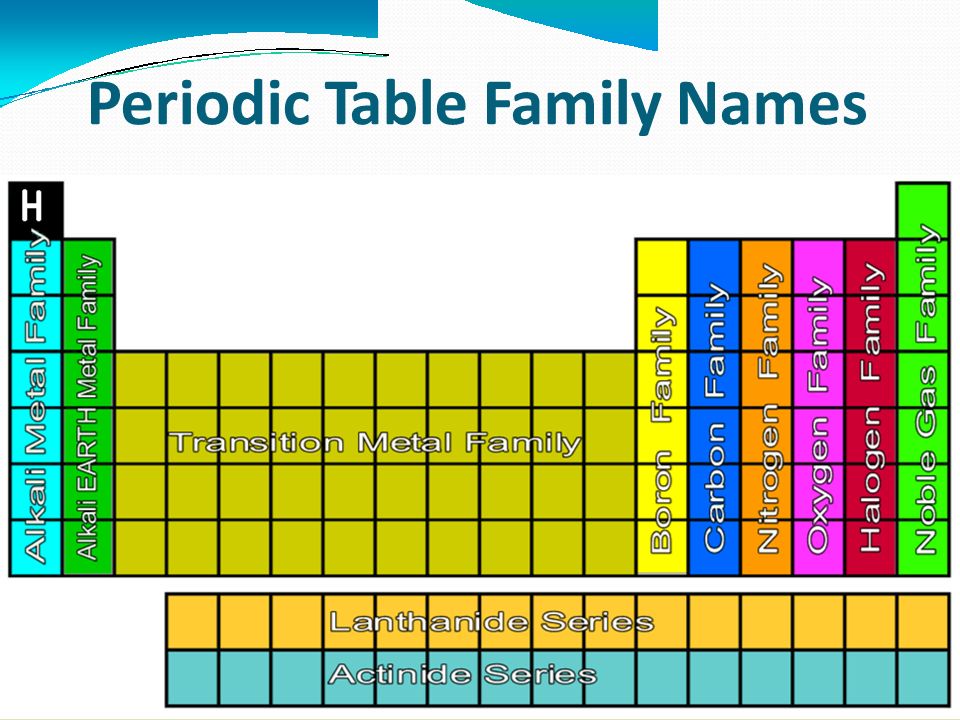
Periodic Table Chemical Families
Following are the important categories of chemical families in a periodic table :
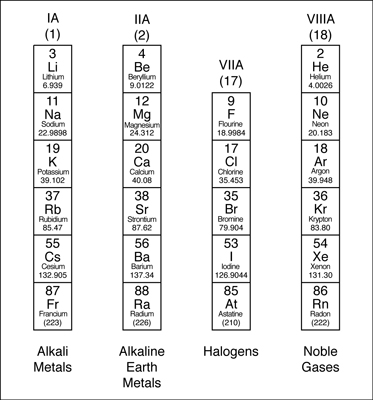
- The group 1A is made of alkali metals
- The group IIA is made up of alkaline earth metals
- The group VIIA is made up of halogens
- The VIII A group is made up of noble gases.
What Is A Period In The Periodic Table
A period in a periodic table is the horizontal row across spanning from left to right. It contains elements which have the same number of electronic shells in a period. So the elements belonging to the same period have similar chemical properties.
For example, alkali metals belong to the first period and show common chemical property of high reactivity and each of the successive element is less metallic than the next element in the periodic table.
lovely periodic table
Yes, Sly Cooper agrees
Mmm, yes gentlemen i agree lovely day we are having.