The particular or specific patterns which represent various specific things in a periodic table are known as periodic trends. These periodic trends illustrate the aspects of various elements which include radius and other electronic properties in the table. The periodic trends are made from the basic arrangement of different elements present in periodic table which also allows the chemist for predicting all properties of any element very easily and quickly without any mistake. As the elements has almost same atomic structure for different elements present in the same period or family and this is the main reason that these trends developed. We all know that nothing is perfect these periodic trends are also having some exceptions like as of ionization energy in Group 3 and 6.
- Hydrogen Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Oxygen Valence Electrons
- Fluorine Valence Electrons
- Neon Valence Electrons
- Sodium Valence Electrons
- Magnesium Valence Electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Phosphorus Valence Electrons
- Sulfur Valence Electrons
- Chlorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Iron Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
- Germanium Valence Electrons
- Arsenic Valence Electrons
- Selenium Valence Electrons
- Bromine Valence Electrons
- Krypton Valence Electrons
- Rubidium Valence Electrons
- Strontium Valence Electrons
- YttriumValence Electrons
- Zirconium Valence Electrons
- Niobium Valence Electrons
- Molybdenum Valence Electrons
- Technetium Valence Electrons
- Ruthenium Valence Electrons
- Rhodium Valence Electrons
- Palladium Valence Electrons
Latest Periodic Table Of Labeled And Periodic Table Of Non-Metals.
Periodic Table Trends❤️
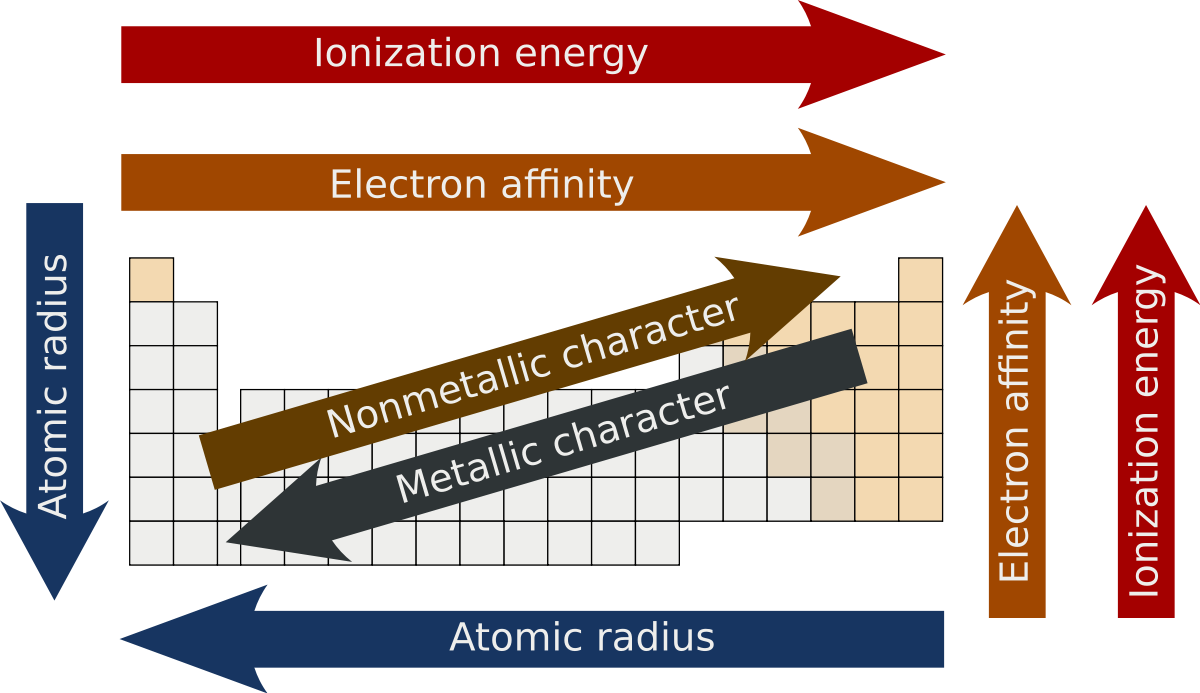
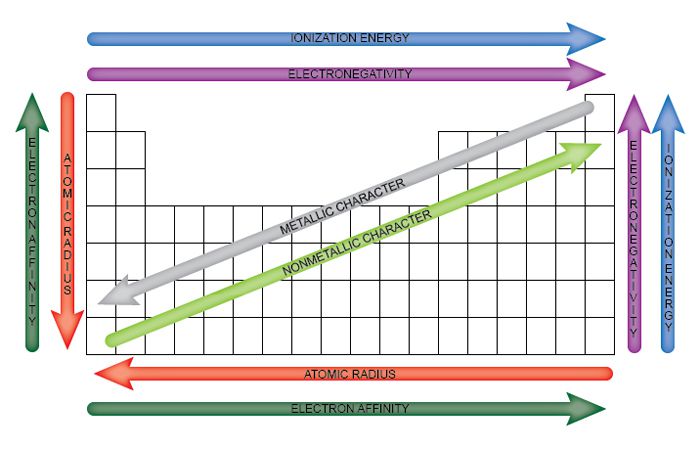
There are lots of trends present in periodic table as here it is not possible to describe them all but we will describe some of them later. In this article we will learn about them but before that let us have the list of these trends and come to know which are the basic periodic trends of a table.
You can also download the printable periodic table Elements with Name.
- Electronegativity
- Ionization Energy
- Atomic Radius
- Electron Affinity
- Ionic Radius
- Atomic Radius
- Metallic Character
- Reactivity
Periodic Law
These trends of the periodic table are reliable on the periodic law, these states that if any chemical element is marked with the increasing order of atomic number, the properties of these changes goes with a cyclic way to represent correct order. Let us have an example for it, like as we are counting two different elements Sodium and Lithium which are having different properties and after arranging them in order of their increasing atomic number some of the chemical and physical properties of these elements recurred like as of reactivity with water and then it again get recurred with other element of next cycle which is Potassium.
For Periodic Table Valency Of Elements And Periodic Table Charges Of Elements.
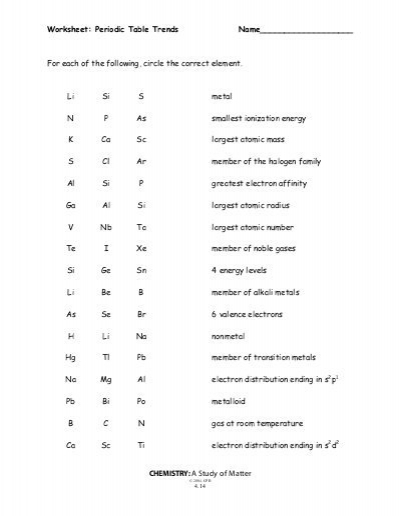
Periodic Table Trends Worksheet
In this section we are going to provide you with a free worksheet for periodic table trends. In this worksheet you can practise each and every trend for all elements present in the periodic table. So, go through the worksheet and gather more knowledge about these tends.
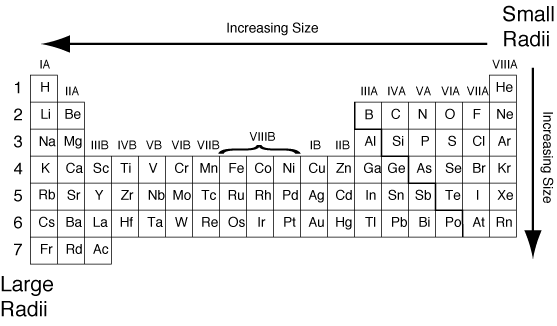
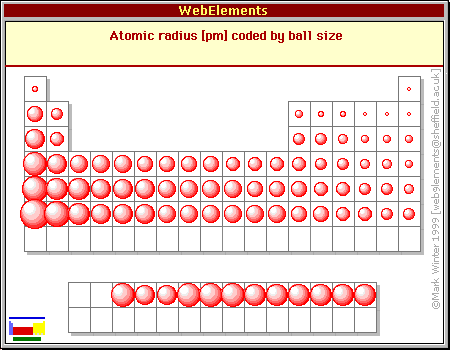
Periodic Table trends Atomic Radius
Atomic radius is one of the trend for the elements of periodic table. For getting to know what is atomic radius and how is it measured we must known that it is the measurement of the distance from the nucleus of an atom to the outermost shell where last electron is present. This trend is found to get decreased when we move left to right across the period and the atomic radius trend usually increase when we move down towards the group. It is also said that the atomic radius increases diagonally as the number of electrons has a great effect over the size of an atom. In lithium with size 145 picometer, the atomic radius is small as compared to the another element magnesium which has atomic size of 150 picometer.
There are different types in which we can categories the atomic radius these are :
1) Van der Waals radius – This type of atomic radius is defined as the half of the distance present in between the different nucleus of atoms available in a lattice formed with the covalent molecules.
2) Ionic Radius – This radius is defined as the half of the distance present in between of two nucleus.
3) Covalent Radius – The covalent radius can be defined as the half of the distance available in between the atoms of a diatomic compound which are bonded singly with each other.
4) Metallic Radius – It is defined as the half of the distance present in between of two adjacent nucleus of different atoms marked up within a metallic lattice.
So, these are different types of atomic radius and its periodic table trend which tells you its effect when moving left to right and top to down in a period or group respectively.
In this article we come to learn about the different trends of periodic table, it is very important to use these trends because they tell us about the correct properties of a particular element and without these trends it will become difficult to tell the property of any element. After learning about periodic table trends we come to know about periodic law, its importance and why we use it during the classification of any element. At last we told you about one of the periodic table trend known as atomic radius this trend is important to tell about the atomic radius of any particular element present within the periodic table. It will let you know which element has bigger size and which one is smaller. There are different types of atomic radius based on the type of atoms and its molecules which we have defined already.
Find Our Blank Periodic Table And Periodic Table Valency Of Oxygen.