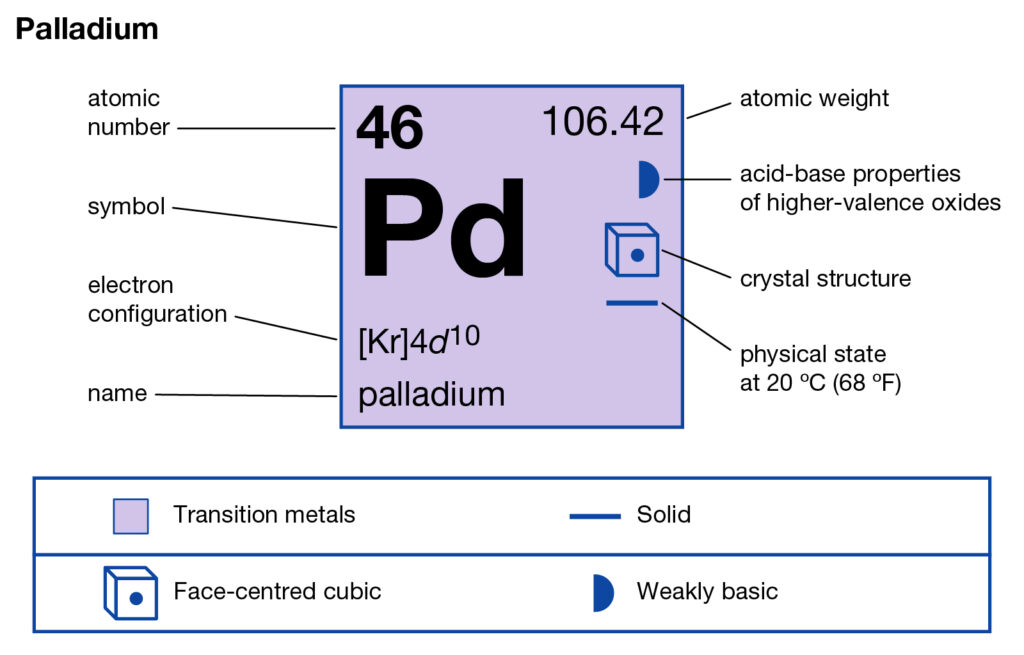
Get to understand the Palladium valence electrons and much more in the article. We shall further disclose the other important properties of this element ahead. In chemistry, Palladium is a well-known chemical element. It has the atomic number 46 and the representative symbol as Pd.
- Flerovium Valence Electrons
- Iron Valence Electrons
- Plutonium Valence Electrons
- Bismuth Valence electrons
- Mercury Valence electrons
- Silicon Valence Electrons
- Lead Valence electrons
- Livermorium Valence Electrons
- Tellurium Valence Electrons
- Radon Valence electrons
- Gold Valence Electrons
- Xenon Valence Electrons
- Nobelium Valence Electrons
- Tennessine Valence Electrons
- Neptunium Valence Electrons
- Radium Valence Electrons
- Moscovium Valence Electrons
- Antimony Valence Electrons
- Titanium Valence Electrons
- Hydrogen Valence Electrons
- Vanadium Valence Electrons
- Boron Valence Electrons
- Chromium Valence Electrons
- Phosphorus Valence Electrons
- Manganese Valence Electrons
- Cesium valence electrons
- Sodium Valence Electrons
- Oganesson Valence Electrons
- Scandium Valence Electrons
- Beryllium Valence Electrons
- Calcium Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Nitrogen Valence Electrons
- Helium Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Aluminum Valence Electrons
- Fluorine Valence Electrons
- Magnesium Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Sulfur Valence Electrons
- Carbon Valence Electrons
- Neon Valence Electrons
How many valence electrons does Palladium have?
The element was first discovered in the year of 1903 by an English chemist. In structure, Palladium looks like a lustrous white silvery metal. Palladium belongs to the Platinum group of elements in the periodic table. It hence closely resembles to Rhodium, ruthenium and Iridium etc elements.
Palladium is least dense than the other elements of the platinum group. It further has the lowest melting point in its category. The element palladium has its free form in nature. You can find it in alloys form with the golds and other platinum group elements.
Russia is the leading producer of Palladium in the present scenario. Africa, the US, and Canada are the other countries to follow Russia in palladium production. Palladium mostly has safe properties, unlike other toxic elements. The element is hence safely usable in the commercial domain as well.
The major use of Palladium is to use it as a catalytic converter. Hence the chemical element has huge relevance in the automobile sectors. Furthermore, Palladium us useful to use in Jewelry, aircraft assembly, and even in the dentistry medical domain. Palladium has a significant availability in nature hence it carries a reasonable price range.
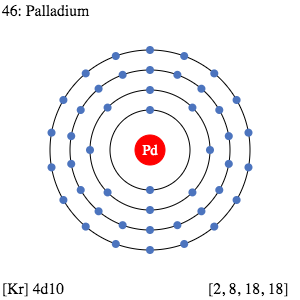
Palladium Valence Electrons Dot Diagram
Valence electrons are the significant properties of Palladium or any other chemical element. We hence have the dot diagram here to simplify the Palladium valence electrons. The diagram reveals the numbers of Pd valence electrons. It draws the dots around the symbol of Palladium.
The dots are equivalent to the numbers of valence electrons. You can further understand the bonding pattern of Palladium valence electrons with the dot diagram.
Valency of Palladium
Palladium has the valency of 2 as its combining capacity. The chemical element has 2 valence electrons in its outer shell hence it has same valency.