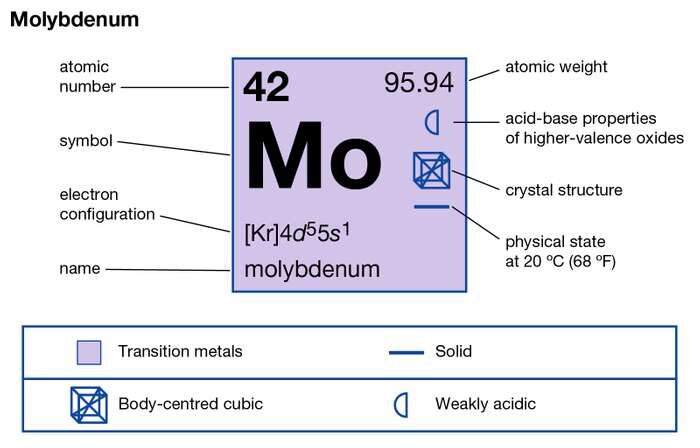
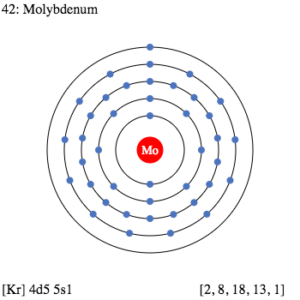
Study the Molybdenum valence electrons here and know the element in a better manner. We are further compiling more useful information on the element in the article. In chemistry, Molybdenum is a chemical element with its atomic number 42. It has the representative symbol as Mo.
- Flerovium Valence Electrons
- Iron Valence Electrons
- Plutonium Valence Electrons
- Bismuth Valence electrons
- Mercury Valence electrons
- Silicon Valence Electrons
- Lead Valence electrons
- Livermorium Valence Electrons
- Tellurium Valence Electrons
- Radon Valence electrons
- Gold Valence Electrons
- Xenon Valence Electrons
- Nobelium Valence Electrons
- Tennessine Valence Electrons
- Neptunium Valence Electrons
- Radium Valence Electrons
- Moscovium Valence Electrons
- Antimony Valence Electrons
- Titanium Valence Electrons
- Hydrogen Valence Electrons
- Vanadium Valence Electrons
- Boron Valence Electrons
- Chromium Valence Electrons
- Phosphorus Valence Electrons
- Manganese Valence Electrons
- Cesium valence electrons
- Sodium Valence Electrons
- Oganesson Valence Electrons
- Scandium Valence Electrons
- Beryllium Valence Electrons
- Calcium Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Nitrogen Valence Electrons
- Helium Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Aluminum Valence Electrons
- Fluorine Valence Electrons
- Magnesium Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Sulfur Valence Electrons
- Carbon Valence Electrons
- Neon Valence Electrons
How many valence electrons does Molybdenum have?
Molybdenum is basically found in nature in its mineral form. Later the separation of Molybdenum is made to attain the pure form of this chemical element. Molybdenum has its first discovering history from the year of 1778. So, Molybdenum has no free form in nature, unlike the other chemical elements.
The chemical element has the appearance of hard silvery and grayish metal. It’s a transition metal but it has no visible reaction with water or oxygen. Molybdenum has no as such significant usages since it has very limited applications. Nearly 90% of the Molybdenum goes in the domain of metal science and engineering.
Further, the rest 10% is useful in chemical applications in the industrial domain. For instance, Molybdenum is useful in the production of alloys. Molybdenum further has anti-corrosion properties as well which makes it the solid resistant element to heat. It is hence readily usable in heat resisting appliances.
Molybdenum Valence Electrons Dot Diagram
You can consider studying the Mo valence electrons with its dot diagram. The diagram draws and represents the actual numbers of valence electrons.
Having the proper understanding of Molybdenum valence electrons will help in understanding the chemical bonding. So, the Lewis dot diagram is very useful for analyzing Molybdenum valence electrons.
Valency of Molybdenum
Well, Molybdenum is one of those elements which have high variable valencies. The element may have the valencies of 2,3,4,5,6 in different scenarios.
It’s a transition chemical element hence it may have variable valency. You can also study the valency of Molybdenum in the periodic table.