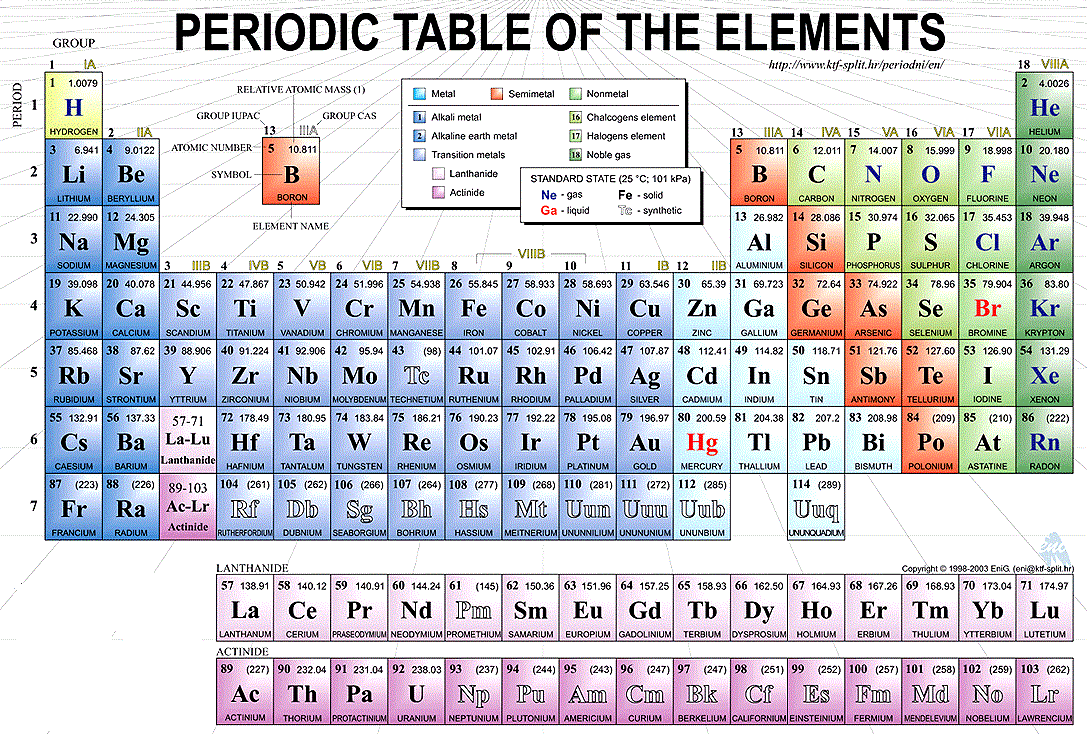
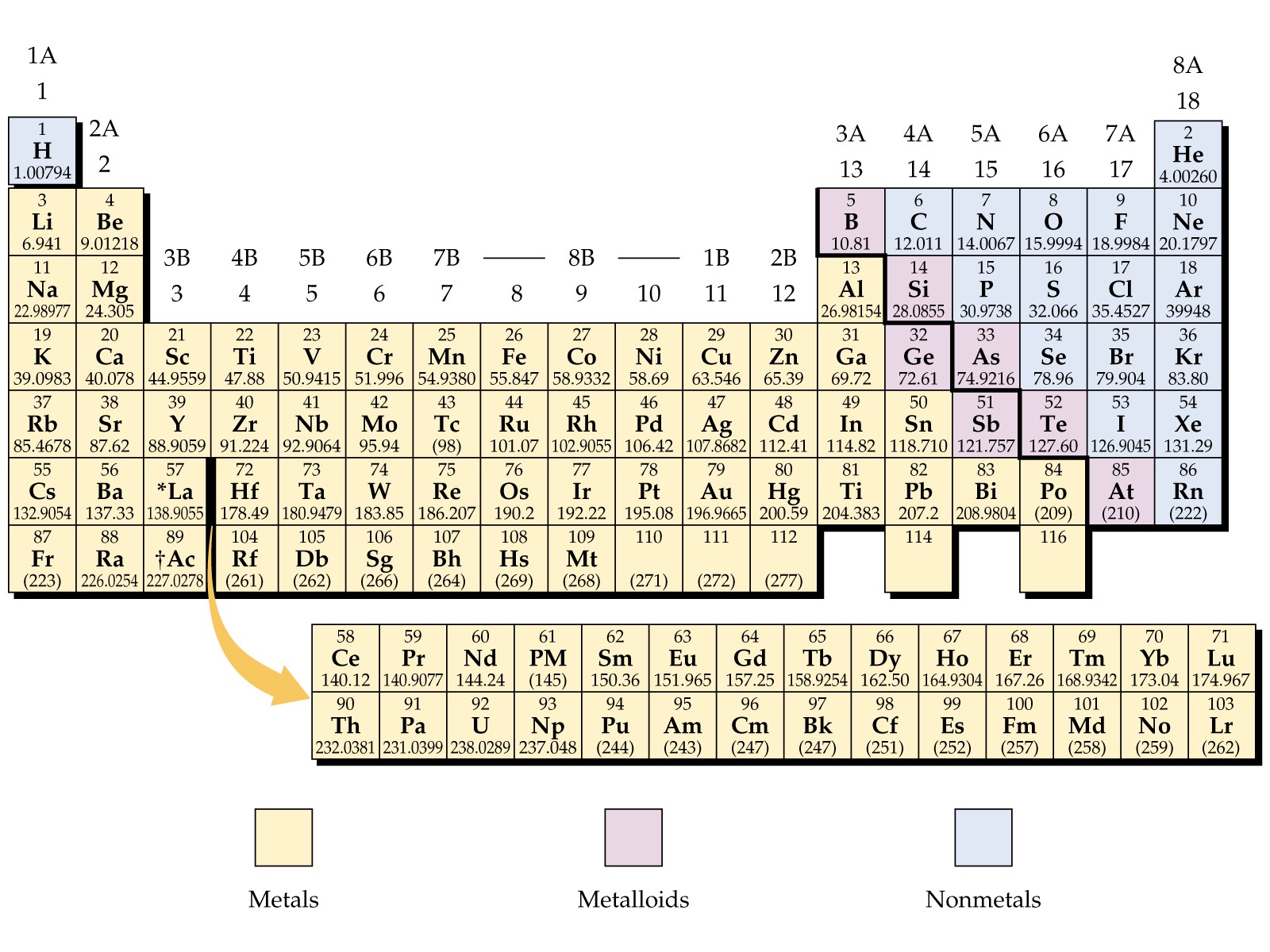
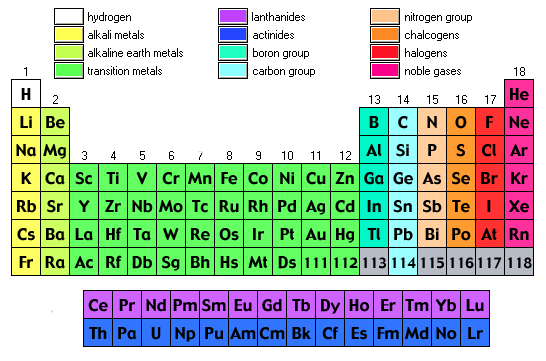
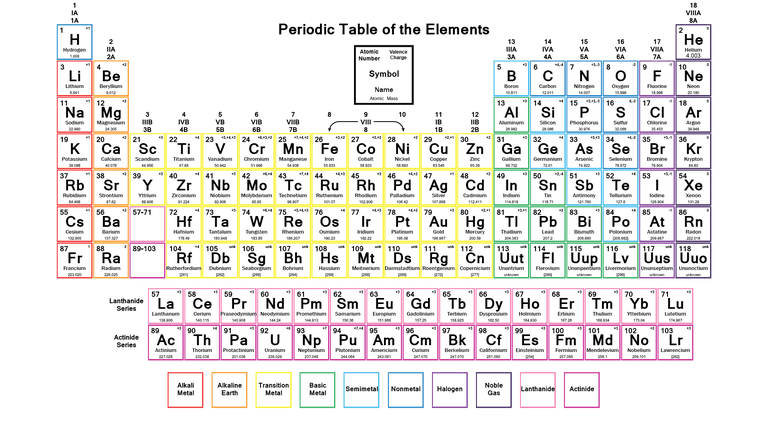
The Periodic Table is the table which arranges the chemical elements in a systematic form that is in a tabular form. The elements are arranged from left to right in order of their increasing atomic number. The atomic number is the number of protons and neutrons present in the nuclei of an element.
Check out here also for valence Electrons:-
- Hydrogen Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Oxygen Valence Electrons
- Fluorine Valence Electrons
- Neon Valence Electrons
- Sodium Valence Electrons
- Magnesium Valence Electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Phosphorus Valence Electrons
- Sulfur Valence Electrons
- Chlorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Iron Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
- Germanium Valence Electrons
- Arsenic Valence Electrons
- Selenium Valence Electrons
- Bromine Valence Electrons
- Krypton Valence Electrons
- Rubidium Valence Electrons
- Strontium Valence Electrons
- YttriumValence Electrons
- Zirconium Valence Electrons
- Niobium Valence Electrons
- Molybdenum Valence Electrons
- Technetium Valence Electrons
- Ruthenium Valence Electrons
- Rhodium Valence Electrons
- Palladium Valence Electrons
The horizontal rows of the periodic table are called periods while vertical columns are known as groups. Elements are classified into different groups depending upon their own characteristics of physical and chemical properties
Labeled Periodic Table of Elements
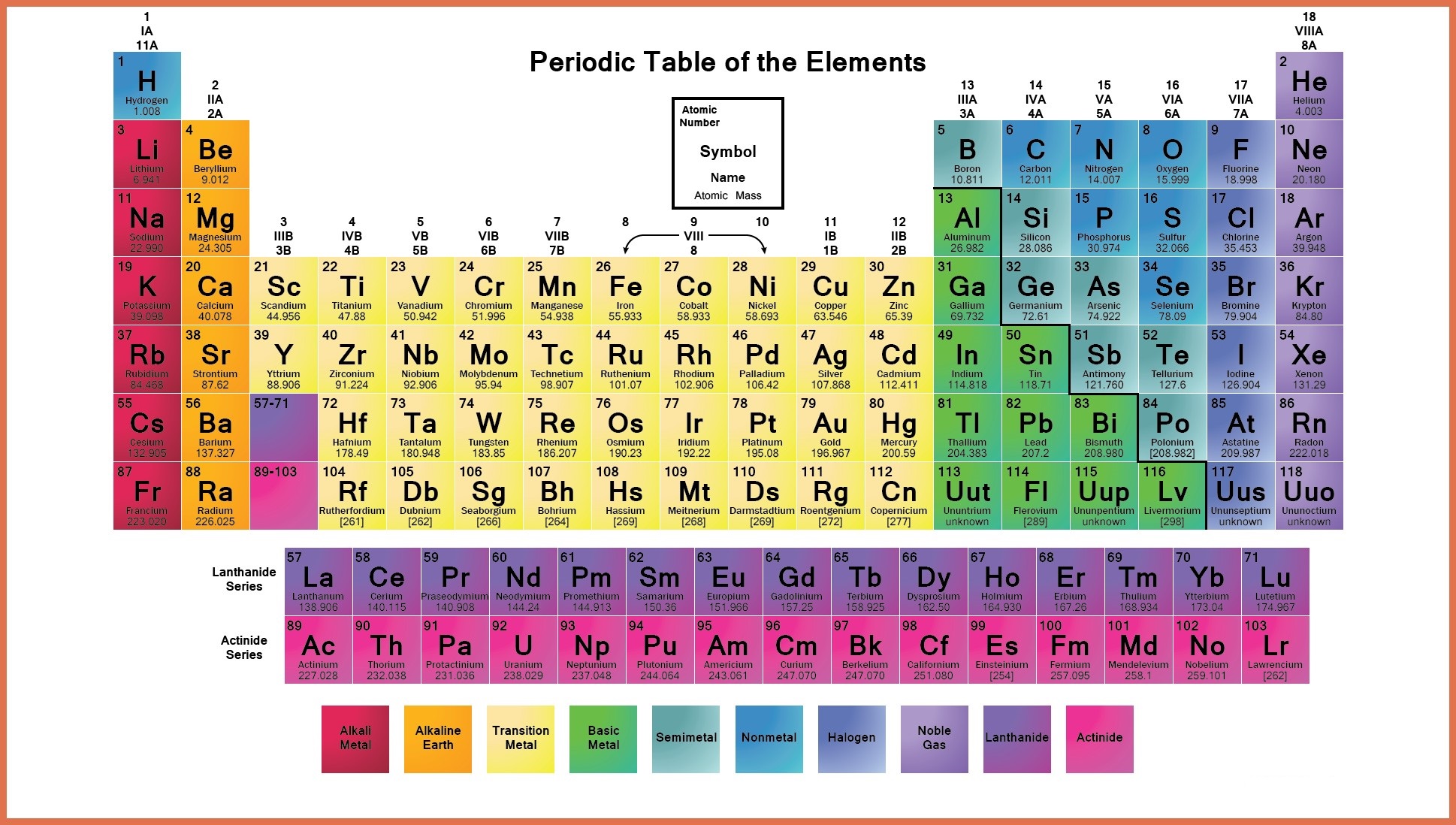
There are different types of elements in a periodic table;
- Metalloids: The elements that come under this group have the characteristic of both metals and non-metals. For example Antimony, Boron, Germanium, Silicon, Arsenic, Tellurium and Polonium
- Alkali Metals: These are placed in the first group of the table and include elements Lithium, Sodium, Potassium, Rubidium, Cesium, Francium.
- Alkaline Earth Metals: These type of metals are placed in the second group of the periodic table, for example, Magnesium, Beryllium, Strontium, Calcium, Barium, and Radium
- Transition Metals: These are transition metals. Malleability, Ductility, and conductivity of electricity are some of the basic characteristics of these elements, for example, Copper, Nickel, Chromium, Mercury, Gold and Silver
- Rare Earth Elements: Such elements are located in the 3rd, 6th and 7th periods of the table. These are mostly man-made for example Curium, Cerium, Uranium, Europium etc.
- Non-Metals: These metals include Hydrogen, Carbon, Nitrogen, Oxygen, Phosphorus, Sulfur and Selenium
- Noble Gases: Noble gases can be found in an 18th group of the periodic table for examples Neon, Argon, Helium, Krypton, Xenon and Radon.
Find Elements Valency Here
Labeled Periodic Table Metals
The elements which are classified as metals in the periodic table share a number of properties. All metals are solid at room temperature except Mercury, which is liquid at room temperature. All metals conduct have property heat and electricity, and they are shiny in appearance. All metals can be changed to thin wires and sheets, this property is called ductility and malleability. Apart from these properties metals can also be characterized by their ability to easily lose electrons.
For Electronegativity Chart and Blank Periodic Table chart visit here.
Periodic Table Labeled Groups
The periodic table labeled with groups is attached below;
Labeled Periodic Table with Charges
The periodic table provided below is labeled with charges, if you want it you can download it from below;
Find Charges Of Elements Here
Latest Periodic Table Trends.