Iodine Electron Configuration: Iodine is a chemical element that has a symbol I. The atomic number of Iodine is 53. It is the heaviest of the stable halogens. It exists as a purple-black, lustrous, non-metallic solid at favorable conditions which readily sublimes to form a violet gas.
- Technetium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Germanium Electron Configuration
- Arsenic Electron Configuration
- Selenium Electron Configuration
- Bromine Electron Configuration
- Krypton Electron Configuration
- Rubidium Electron Configuration
- Strontium Electron Configuration
- Yttrium Electron Configuration
Iodine Electron Configuration
The elemental form of Iodine was discovered by Bernard Courtois, a French chemist in 1811. Iodine was named two years later by Joseph-Louis Gay-Lussac from this property. It is found in many oxidation states which includes iodide and iodate and many other various periodate anions.
- Hydrogen Valency
- Helium Valency
- Lithium Valency
- Beryllium Valency
- Boron Valency
- Carbon Valency
- Nitrogen Valency
- Oxygen Valency
- Fluorine Valency
- Neon Valency
- sodium Valency
- Magnesium Valency
- Aluminum Valency
- Silicon Valency
- Phosphorus Valency
Iodine is the least abundant of the stable halogens and also the sixty-first most abundant element. Today we are going to give you all the information related to the electron configuration of the Iodine.
Iodine Number of Valence Electrons
Iodine has seven Valence Electrons.
What is The Electron Configuration of Iodine
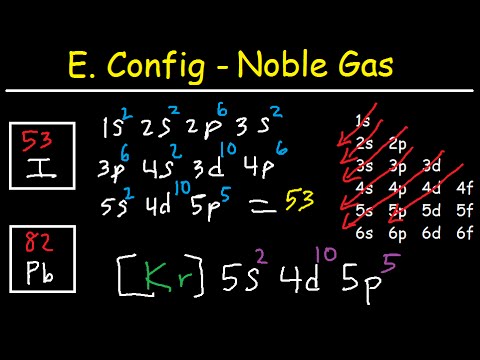
There are 53 electrons in iodine that occupy the respective orbitals as given below;
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵
As 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ is the electronic configuration of the previous noble gas Krypton, Kr, so we write it like
[Kr] 5s² 4d¹⁰ 5p⁵
You can also see the image below
How Many Valence Electrons Does Iodine Have
There are 7 valence electrons with Iodine.