Yttrium Electron Configuration: Yttrium chemical element is one of the rarest chemical elements available on the earth. This chemical element is very highly toxic and you can’t find it as a free element in nature’s lap, unlike many other chemical elements.
Today in this article we are going to discuss the electron configuration of this rare chemical element.
- Cobalt Electron Configuration
- Nickel Electron Configuration
- Copper Electron Configuration
- Chlorine Electron Configuration
- Argon Electron Configuration
- Potassium Electron Configuration
- Scandium Electron Configuration
- Titanium Electron Configuration
- Vanadium Electron Configuration
- Nickel Electron Configuration
- Zinc Electron Configuration
- Germanium Electron Configuration
- Arsenic Electron Configuration
- Selenium Electron Configuration
- Bromine Electron Configuration
Yttrium Electron Configuration
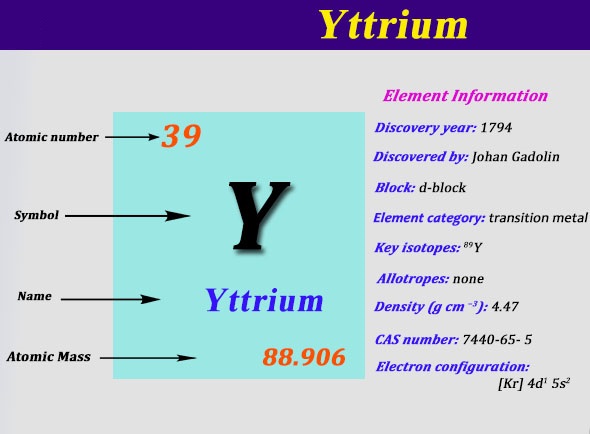
Yttrium is basically a chemical element which is denoted by the symbol of Y and in the chemistry, the atomic number of Yttrium is 39. This element is often considered to be a rare element of the earth.
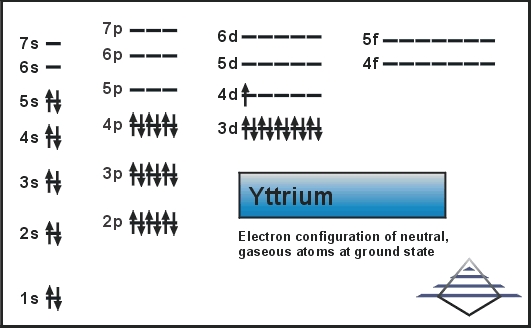
The electron configuration for Yttrium is basically a process in which the electron of the Yttrium chemical element is distributed in the molecular orbital. This whole process of Electron configuration helps the scientists or the chemistry field researcher to understand the chemical reaction of any element.
What is the Electron Configuration of the Yttrium?
The chemical element Yttrium is having 39 as its atomic number and is located in the group 3 of the periodic table. The electron configuration of the Yttrium can be written as [Kr] 4d1 5s2
This chemical element is mostly known for its significant contribution to the production of the phosphorus and from there it is used in mobile phones which are being manufactured at a massive scale in the present scenario.
How Many Valence Electrons Does Yttrium Have?
Well, that’s an interesting question and for your answer, the Valence of the Yttrium element is 2. Yes, it holds 2 valences which are 0 in the shape of metal and the +3 as a chemical combination.
Yttrium is best found in Asian countries such as India, China, Malaysia etc. This chemical element is used widely in mobile phones and in the picture tube of television.
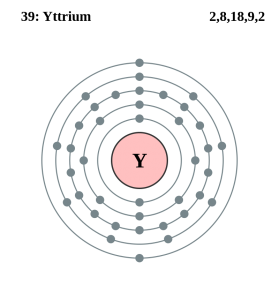
Yttrium Number of Valence Electrons
Yttrium chemical is the element, which has an atomic number of 39 and known to be one of the rarest elements on the earth. Yttrium chemical element has 39 protons and 50 neutrons. It is basically a transition metal that is located in group 3 and period 5.
Leave a Reply