Learn about the Yttrium valence electrons here and deepen your chemistry knowledge. You can learn about all the relevant properties of Yttrium in the article. Yttrium is the chemical element as per the chemistry branch of science. It has an atomic number of 39 and the representative symbol of Y.
- Flerovium Valence Electrons
- Plutonium Valence Electrons
- Mercury Valence electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neptunium Valence Electrons
- Moscovium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Iron Valence Electrons
- Cesium valence electrons
- Bismuth Valence electrons
- Silicon Valence Electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Xenon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Hydrogen Valence Electrons
- Phosphorus Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Sodium Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Helium Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Fluorine Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Aluminum Valence Electrons
- Magnesium Valence Electrons
- Neon Valence Electrons
- Sulfur Valence Electrons
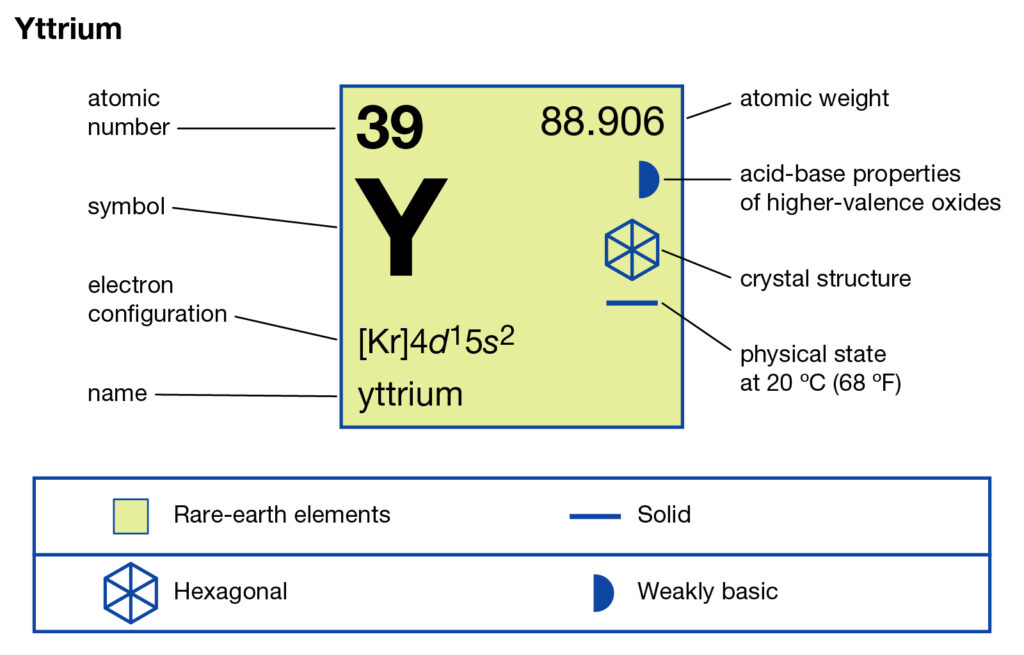
How many valence electrons does Yttrium have?
There is no free form of Yttrium in the crust of earth just like the other chemical elements. It occurs or takes place with the combination of lanthanide which is a rare earth mineral. Yttrium has the narrative as the rare earth metal due to its complex derivation. It has its discovery origin in Sweden in 1787.
Yttrium is one of the most useful chemical elements from the commercial industry’s perspective. It has direct and significant use in modern LED’s, Phosphorus, etc. Red phosphorus is useful in the production of old styled cathode ray tube televisions. There are other users of Yttrium such as in the production of lasers, conductors, electronic filters, etc.
Other than the commercial usage Yttrium has no proven biological usage. In fact, human exposure to Yttrium can cause serious illness of the liver. So, in conclusion, Yttrium is a significant chemical element in the commercial domain. Further scientific research is going on for its more proven usages in other domains.
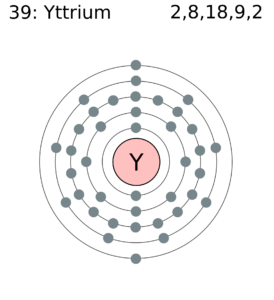
Yttrium Valence Electrons Dot Diagram
You can explore the better picture of Yttrium valence electrons with the dot diagram. The diagram draws the dots around the symbol of Y which is the actual number of valence electrons. Further, the dot diagram is also helpful in understanding the type of bonding for the valence electrons. If it’s single bonding then there will be pair of dots around the symbol.
In a similar manner, the double pair of dots will depict the double bonding. So, dot diagram helps in revealing the comprehensive properties of Yttrium.
Valency of Yttrium
Yttrium has three valence electrons in its outer shell. So, it has the 3 valence electrons as its combining capacity.
Leave a Reply