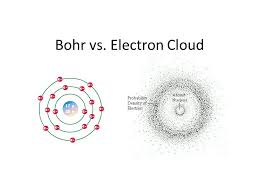
Electron cloud model was given by Erwin Schrödinger and Werner Heisenberg in 1925. Through this model, they tried to explain the probable positioning of an atom. According to this model, electrons can be found at certain places in clouds near the neutrons.
As you know, atoms are the smallest constituent of matter that is present in the universe. The general structure of an atom consists of a positively charged neutron surrounded by the negatively charged electrons.
- Hydrogen Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Oxygen Valence Electrons
- Fluorine Valence Electrons
- Neon Valence Electrons
- Sodium Valence Electrons
- Magnesium Valence Electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Phosphorus Valence Electrons
- Sulfur Valence Electrons
- Chlorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Iron Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
- Germanium Valence Electrons
- Arsenic Valence Electrons
- Selenium Valence Electrons
- Bromine Valence Electrons
- Krypton Valence Electrons
- Rubidium Valence Electrons
- Strontium Valence Electrons
- YttriumValence Electrons
- Zirconium Valence Electrons
- Niobium Valence Electrons
- Molybdenum Valence Electrons
- Technetium Valence Electrons
- Ruthenium Valence Electrons
- Rhodium Valence Electrons
- Palladium Valence Electrons
Since the negative charge of electrons and the positive charge of the protons are opposite but equal, they balance out each other and the net result is that an atom remains neutral. But in some cases, it may happen that during chemical reactions, an atom may lose or gain an electron. If it loses an electron, it becomes negatively charged and is known as an anion. If it gains an electron, it becomes positively charged an is known as a cation.
Examples of anions and cations are sodium and chlorine atoms respectively.
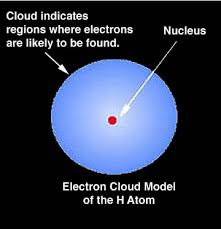
Electron Cloud Model Example
According to the Bohr’s calculation for a hydrogen atom, the electron under normal conditions always stays at a distance of 0.529Å from the nucleus.
But according to the cloud model, the electron keeps on moving away or towards the nucleus and the maximum probability of locating it lies at a distance of 0.529Å from the nucleus. So, to conclude, the radius of electron cloud or radius of maximum probability is 0.529Å.