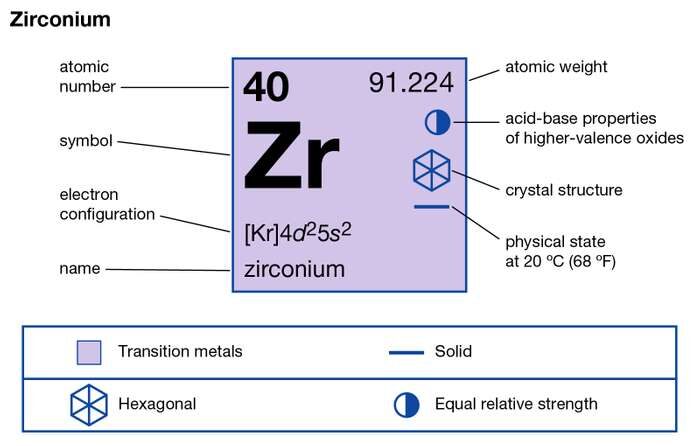
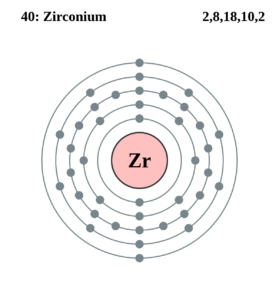
Get all the facts and information about Zirconium valence electrons here. The article will enhance your knowledge about this particular chemical element. Zirconium is a chemical element with its atomic number of 40. It has the symbol of Zr and it owes its name to the mineral Zircon.
- Flerovium Valence Electrons
- Plutonium Valence Electrons
- Mercury Valence electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neptunium Valence Electrons
- Moscovium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Iron Valence Electrons
- Cesium valence electrons
- Bismuth Valence electrons
- Silicon Valence Electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Xenon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Hydrogen Valence Electrons
- Phosphorus Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Sodium Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Helium Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Fluorine Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Aluminum Valence Electrons
- Magnesium Valence Electrons
- Neon Valence Electrons
- Sulfur Valence Electrons
How many valence electrons does Zirconium have?
The structure of this element is hard lustrous grayish or white metal. It belongs to the category of transition metals and resembles hafnium. Zirconium is basically extracted as the byproduct of Titanium and Tin mining. The metal is basically more expensive than the Zircon itself due to its processing cost.
Zirconium is basically a purely commercial product as it has plenty of usages around. Furthermore, a small proportion of Zirconium is useful in other metals production as well. With its anti-corrosion properties Zirconium is useful in the alloy production. The refractory properties of the element make it the best resistance of heat in the number of equipment.
Zirconium Valence Electrons Dot Diagram
Furthermore, there are many other usages of Zirconium in several other domains. For instance, you can see its application in nuclear researches or even in the medical domain as well.
We have the Zirconium dot diagram here to provide the systematic study of its valence electrons. The diagram will guide you in understanding the chemical reaction of Zr valence electrons. You can further witness the type of chemical bonding reaction as to whether it’s single or double bonding. The pair of dots convey the single bonding while the double pair means double bonding.
Valency of Zirconium
The valency of Zr is 4 valence electrons in its outer shell. The numbers of valence electrons in the outer shell of zirconium are exactly its valency. The valency of zirconium is useful in figuring out the combining capacity of elements. You can also refer to the periodic table to cross-check Zirconium’s valency