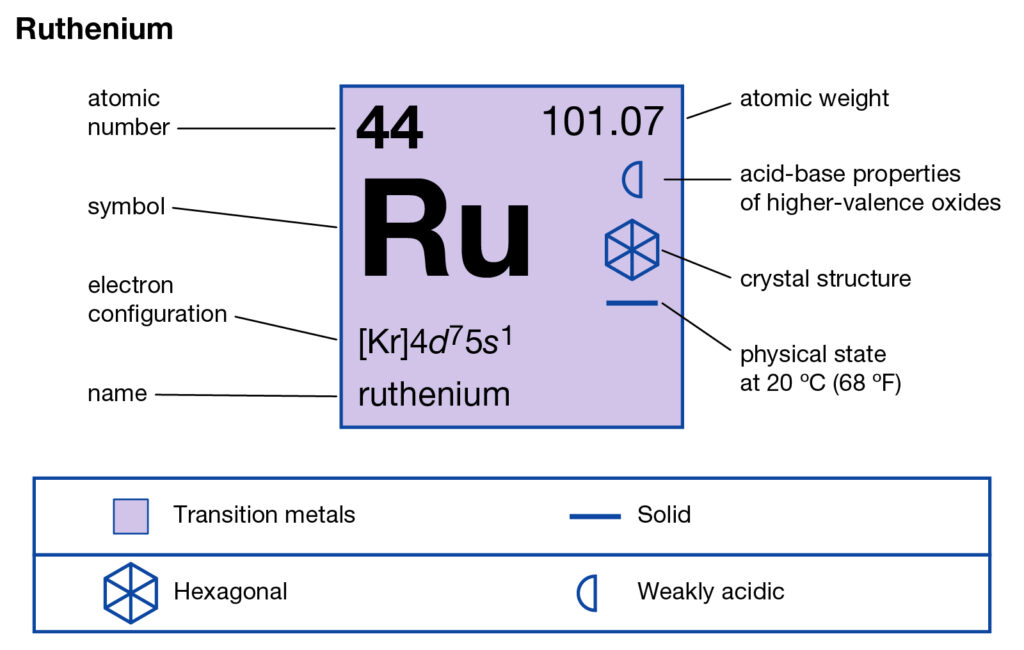
We are going to compile the systematic study of Ruthenium valence electrons here. The article will further provide other relevant information of elements. Ruthenium is a newly emerged chemical element in the chemistry branch of science. The element has the atomic number as 44 and the symbol of Ru.
- Flerovium Valence Electrons
- Iron Valence Electrons
- Plutonium Valence Electrons
- Bismuth Valence electrons
- Mercury Valence electrons
- Silicon Valence Electrons
- Lead Valence electrons
- Livermorium Valence Electrons
- Tellurium Valence Electrons
- Radon Valence electrons
- Gold Valence Electrons
- Xenon Valence Electrons
- Nobelium Valence Electrons
- Tennessine Valence Electrons
- Neptunium Valence Electrons
- Radium Valence Electrons
- Moscovium Valence Electrons
- Antimony Valence Electrons
- Titanium Valence Electrons
- Hydrogen Valence Electrons
- Vanadium Valence Electrons
- Boron Valence Electrons
- Chromium Valence Electrons
- Phosphorus Valence Electrons
- Manganese Valence Electrons
- Cesium valence electrons
- Sodium Valence Electrons
- Oganesson Valence Electrons
- Scandium Valence Electrons
- Beryllium Valence Electrons
- Calcium Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Nitrogen Valence Electrons
- Helium Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Aluminum Valence Electrons
- Fluorine Valence Electrons
- Magnesium Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Sulfur Valence Electrons
- Carbon Valence Electrons
- Neon Valence Electrons
How many valence electrons does Ruthenium have?
Ruthenium is a transition metal and it belongs to the category of the platinum group. It’s known as the rare earth metal due to its limited availability. It’s basically a byproduct of platinum as it comes out during the extraction process of platinum ores. The element was first discovered in the year of 1844 by the Russian scientist.
The availability of the Ruthenium is gradually increasing along with the plantium. However, the proportion of Ruthenium remains low in the comparison of platinum. South Africa and Russia are the major countries producing the Ruthenium. Presently there is about 30 tonnes of Ruthenium is available on an annual consumption basis.
The major usage of Ruthenium goes to the sector of electrical applications. For instance, most of the solar cells use Ruthenium for electrical contact. Furthermore, the element is useful in the Jewelry domain as well as the alloy with platinum. Ruthenium is toxic in nature hence its exposure to humans is lethal.
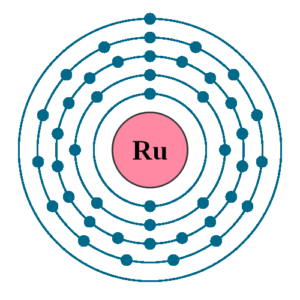
Ruthenium Valence Electrons Dot Diagram
Readers can here analyze the Ruthenium valence electrons with the Lewis dot diagram. The diagram shows the valence electrons numbers around the symbol of Ruthenium.
In a similar manner further, it shows the chemical interaction of Ru valence electrons. You will be able to figure out the combining bonding of valence electrons with a dot diagram.
Valency of Ruthenium
Well, the Ruthenium has the valence as 1,2,3,4,5,6,7,8 respectively. It has a highly variable valency due to its transition properties.