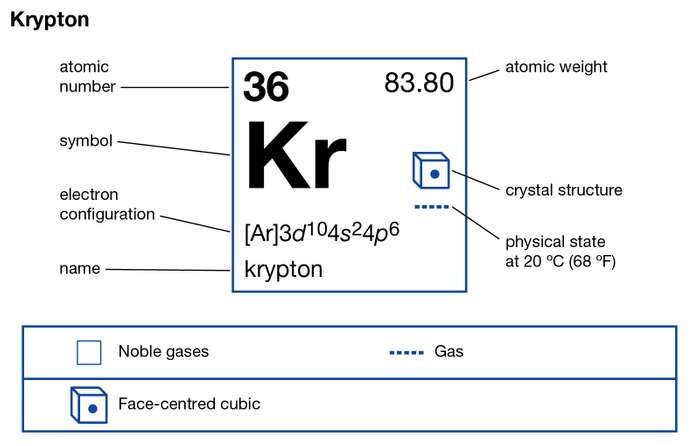
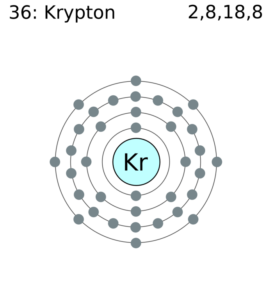
Explore the Krypton valence electrons here and have effective learning of the element. You can also read the other important information of this element in the article. Krypton is a chemical element with the symbol of Kr. It belongs to the noble gas family and has an atomic number of 36.
- Flerovium Valence Electrons
- Plutonium Valence Electrons
- Mercury Valence electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neptunium Valence Electrons
- Moscovium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Iron Valence Electrons
- Cesium valence electrons
- Bismuth Valence electrons
- Silicon Valence Electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Xenon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Hydrogen Valence Electrons
- Phosphorus Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Sodium Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Helium Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Fluorine Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Aluminum Valence Electrons
- Magnesium Valence Electrons
- Neon Valence Electrons
- Sulfur Valence Electrons
How many valence electrons does Krypton have?
Being the noble gas, Krypton has no color, odor or even taste. There is the significant availability of Krypton in the atmosphere of the earth. Krypton is useful to use in integration with other nobles gases in the fluorescent lumps form. Furthermore, Krypton is also usable as the chemical inert form since it has no chemical reaction.
Well, just like the other noble chemical gases Krypton is also useful in the listening form. Krypton plasma is useful in the production of high powered gas lasers.
The typical camera flash also has a significant portion of Krypton. There are a number of lasers around the world that are made of Krypton. Krypton further forms an important part of Insulating gases such as in window panes. There are the other various major usages of Krypton as the pure noble gas.
Krypton Valence Electrons Dot Diagram
You can here understand the interaction and the actual numbers of Krypton valence electrons. We typically use the Lewis dot diagram to unfold the numbers of valence electrons. The diagram basically denotes the several dots around the symbol of Krypton. The numbers of dots are equivalent to the valence electrons.
It further shows the bonding pattern of valence electrons of an atom. You can analyze the proper krypton valence with this dot diagram.
Valency of Krypton
Krypton has the 8 valence electrons in its outer shell hence it has the valency of 8. Krypton remains in the far outer side of periodic table to have such high valency.