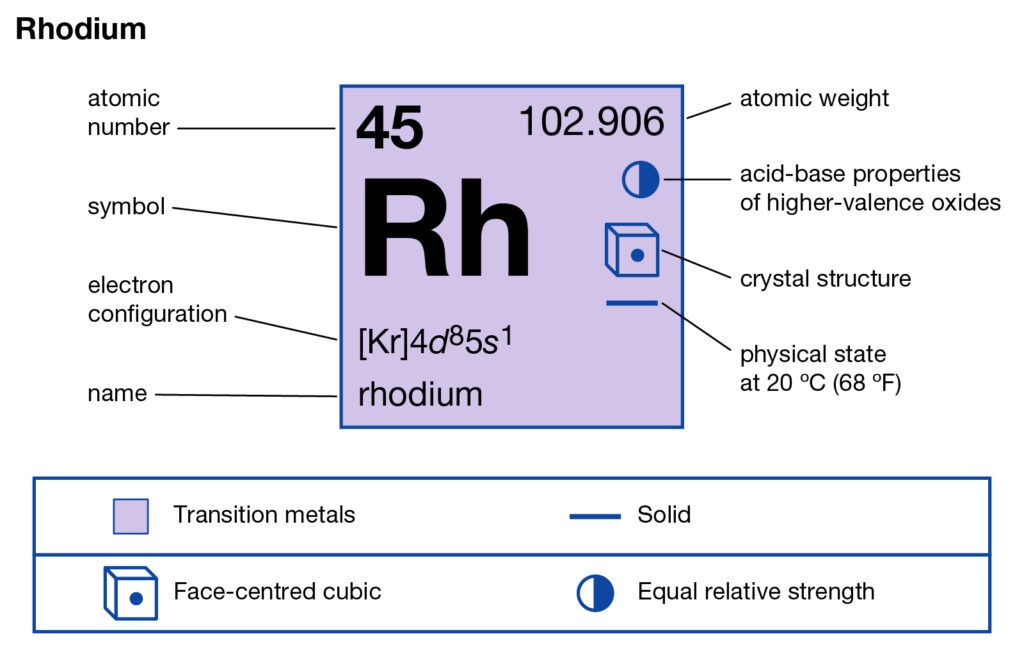
Learn about the Rhodium valence electrons here and enhance your knowledge of elements. The article will guide you in the systematic learning of this element. Rhodium is a chemical element in chemistry. It carries the atomic number 45 and the represents symbol as Rh.
- Flerovium Valence Electrons
- Iron Valence Electrons
- Plutonium Valence Electrons
- Bismuth Valence electrons
- Mercury Valence electrons
- Silicon Valence Electrons
- Lead Valence electrons
- Livermorium Valence Electrons
- Tellurium Valence Electrons
- Radon Valence electrons
- Gold Valence Electrons
- Xenon Valence Electrons
- Nobelium Valence Electrons
- Tennessine Valence Electrons
- Neptunium Valence Electrons
- Radium Valence Electrons
- Moscovium Valence Electrons
- Antimony Valence Electrons
- Titanium Valence Electrons
- Hydrogen Valence Electrons
- Vanadium Valence Electrons
- Boron Valence Electrons
- Chromium Valence Electrons
- Phosphorus Valence Electrons
- Manganese Valence Electrons
- Cesium valence electrons
- Sodium Valence Electrons
- Oganesson Valence Electrons
- Scandium Valence Electrons
- Beryllium Valence Electrons
- Calcium Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Nitrogen Valence Electrons
- Helium Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Aluminum Valence Electrons
- Fluorine Valence Electrons
- Magnesium Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Sulfur Valence Electrons
- Carbon Valence Electrons
- Neon Valence Electrons
How many valence electrons does Rhodium have?
It’s known as the highly rare chemical element due to its minimal availability in nature. So, it belongs to the category of transition metals in the periodic table. Rhodium has the physical structure as the white hard and chemically inert metal. It has the tag as the noble platinum group metal.
Rhodium has its extraction from the nickel and platinum ores. This is why it belongs to the platinum group category in the periodic table. As we know that Rhodium has a very negligible free form which makes it a highly costly element. It is thus one of the most precious chemical elements.
The major use of Rhodium includes as the catalyst in the automobile mechanism. It works as a three-way catalytic converter in automobiles. The other uses of Rhodium include in the domain of ornaments. It’s useful in the gold plating of white gold to make it shiny and lucrative.
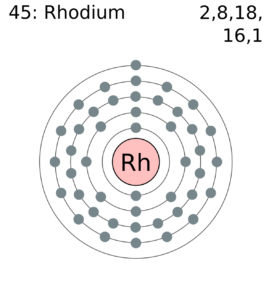
Rhodium Valence Electrons Dot Diagram
We are providing the dot diagram of Rhodium valence electrons. So, you can use this diagram to know the actual number of valence electrons.
In a similar manner, the diagram helps in understanding the interaction of valence electrons. You can further know the valence electrons bonding pattern after the interaction.
Valency of Rhodium
Rhodium has the valency of 3 and 21 valence electrons in its outer shell. You can hence refer the valency as the combining capacity of Rhodium. So, valency is useful in having the proper analysis of chemical elements. You can further check the Rhodium valency in the periodic table.
Leave a Reply