Periodic Table For Dummies: As we all know that chemistry is all about the chemical elements, which are used as the core properties of the chemistry.There are the numerous types of the chemical elements in the domain of chemistry, which are all the species of the atoms.
- Hydrogen Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Oxygen Valence Electrons
- Fluorine Valence Electrons
- Neon Valence Electrons
- Sodium Valence Electrons
- Magnesium Valence Electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Phosphorus Valence Electrons
- Sulfur Valence Electrons
- Chlorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Calcium Valence Electrons
- Scandium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Iron Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
- Germanium Valence Electrons
- Arsenic Valence Electrons
- Selenium Valence Electrons
- Bromine Valence Electrons
- Krypton Valence Electrons
- Rubidium Valence Electrons
- Strontium Valence Electrons
- YttriumValence Electrons
- Zirconium Valence Electrons
- Niobium Valence Electrons
- Molybdenum Valence Electrons
- Technetium Valence Electrons
- Ruthenium Valence Electrons
- Rhodium Valence Electrons
- Palladium Valence Electrons
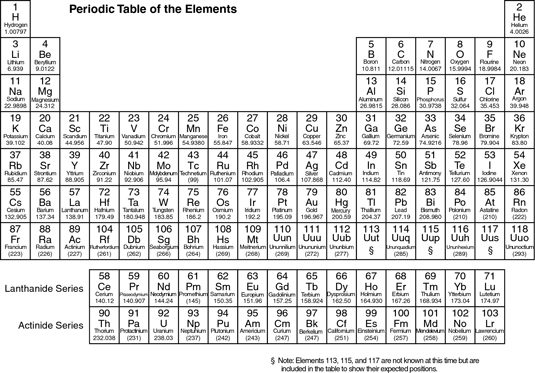
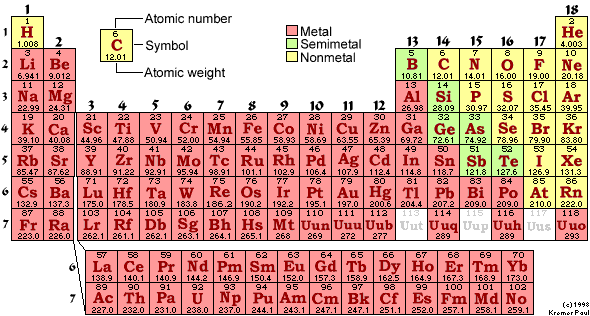
A periodic table is closely linked with the chemical elements as the table contains all the chemical elements which are used in the domain of chemistry. The periodic table provides the tabular presentation of chemical elements along with all other major properties of these chemical elements.
It is like the fundamental book using which you can check out all the chemical properties such as the electron configuration,atomic number, periodic trends etc. The periodic table is comprised of the seven rows in which there are the metals on the left side and the non metals on the right side.
In short if you want to have the proper and the complete understanding of the chemical elements, then it’s the periodic table which you will need to access since in this table all the chemical elements are arranged in a systematic manner just like you arrange all the spices on the shelf of your kitchen in order to prepare a delicious food using them.
So, if you are someone either the scholar of the chemistry or the chemist and are looking for the proper periodic table then here in this article we are going to provide you with the proper understanding of the periodic table.
We are below going to discuss about some of the major compositions of the periodic table, so that you can easily understand all the properties of the chemical elements.
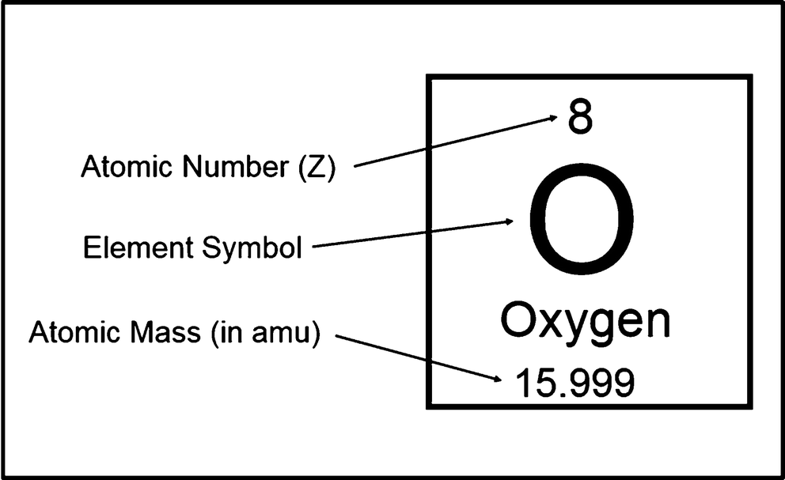
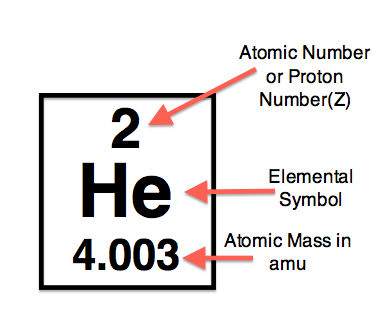
- Atomic Number
It is the first and the most important property of a chemical element as the atomic number is the first characteristic of a chemical element which helps you in identifying the element.
A chemical element may have the low or the high chemical element as the atomic numbers start from the 1 and can reach up to 118 and using the atomic numbers you can easily figure out the other properties of such element for instance the coordination number,valency etc of such element.
2. Proton Numbers
A proton is the significant aspect of any chemical element which can be defined as the positively charged particle and gets counterbalanced with the electrons of such element.
The numbers of the proton for any element determine that how many electrons of that element can orbit around the atoms, which in results affect both the chemistry and the reactivity of such element with the other elements and therefore it is very significant to be understood.
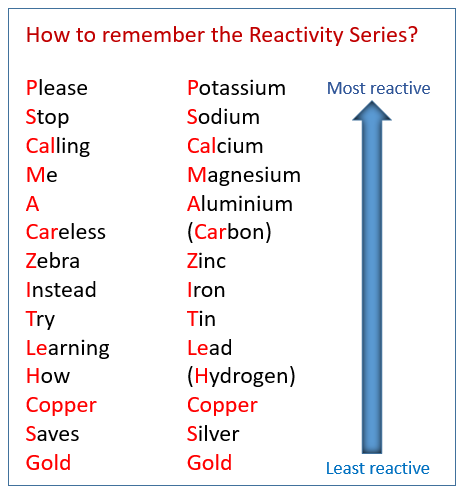
3. Reactivity
Reactivity is basically such property of a chemical element with which it undergoes the chemical reaction with the other chemical elements and then consequently releases the energy.
The chemical reactivity of any element increases when it goes from the left to right within the periodic table, which means any element moving from the left to right side tends to be more reactive and vice versa.
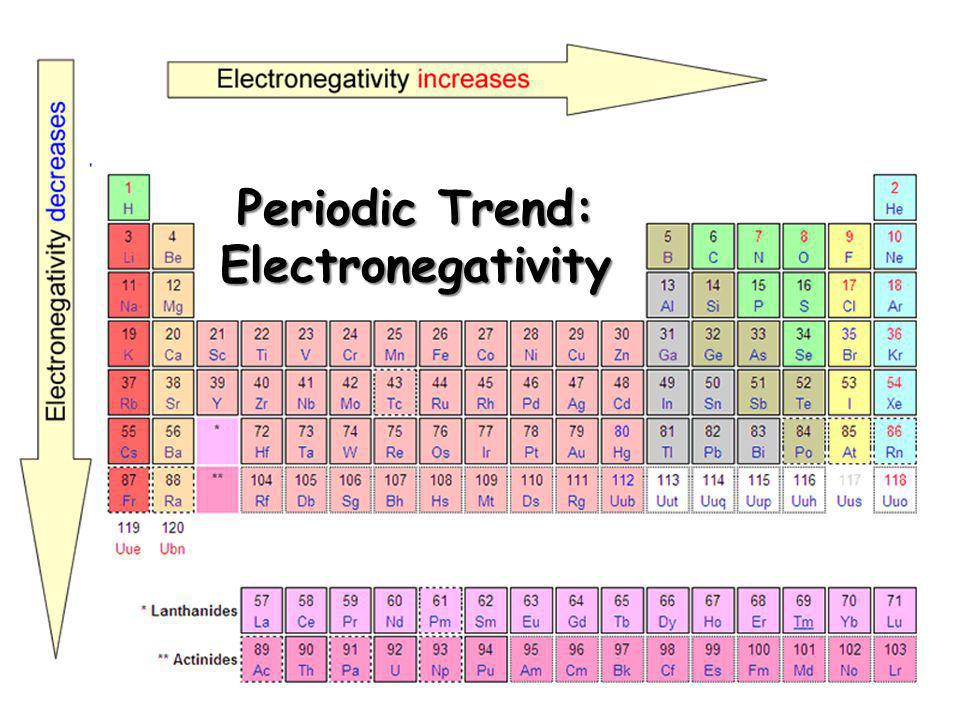
4. Electronegativity
Well, the electronegativity of a chemical element is more or less just like the chemical reaction. Electronegativity basically implies the power of a chemical element with which it attracts the electrons of the other chemical elements.
Using this property you can check out the reactivity of any chemical element since the more reactive element is always going to attract more electrons.
5. Mass
When any chemical element makes a move from the left to the right and from the top to bottom it always tends to accumulate the more protons in the atom and due to this trend the latter element becomes heavier than the former, which is known to be the mass of such chemical element.
It is just the general trend which has its applicability subject to many exceptions but for the basic understanding you can take it in the same sense.
5. Density
When a chemical element goes down to the table from the top then it tends to be more dense due to the increased density. In the similar manner when the element makes move from left to right the density increases in the same manner.
So, these are the major components of a chemical element which you are going to get in the periodic table. All the above mentioned properties of the chemical elements are mandatory in nature and a chemical researcher or the scholar must have the proper understanding of all these properties, in order to understand the chemical element in a right manner.
Leave a Reply