Helium Valence Electrons: Helium is a chemical element of the periodic table. It is the 2nd lightest element out of all other elements. It is a type of gas that converts in a liquid stage at 268.9°C. Also, it is odorless, tasteless, and colorless. It’s boiling and freezing points are lower than the other existing substance.
- Flerovium Valence Electrons
- Mercury Valence electrons
- Moscovium Valence Electrons
- Bismuth Valence electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Oganesson Valence Electrons
- Tellurium Valence Electrons
- Nobelium Valence Electrons
- Xenon Valence Electrons
- Neptunium Valence Electrons
- Cesium valence electrons
- Plutonium Valence Electrons
- Iodine Valence Electrons
- Americium Valence Electrons
- Radium Valence Electrons
- Gold Valence electrons
- Lead Valence electrons
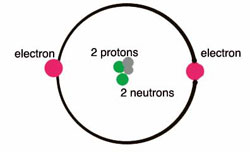
It is an element that cannot be solidified at any temperature. Helium gas is made up of natural gas with the liquid form of other components in low temperatures and under high pressures.
- Hydrogen Valency
- Helium Valency
- Lithium Valency
- Beryllium Valency
- Boron Valency
- Carbon Valency
- Nitrogen Valency
- Oxygen Valency
- Fluorine Valency
- Neon Valency
- sodium Valency
- Magnesium Valency
- Aluminum Valency
- Silicon Valency
- Phosphorus Valency
- Sulfur Valency
- Clorine Valency
- Argon Valency
- Potassium Valency
- Calcium Valency
- Titanium Valency
- Vanadium Valency
- Chromium Valency
- Manganese Valency
- Cobalt Valency
- Nickel Valency
How many valence electrons does helium have?
There are a total of 2 helium valence electrons. This means two helium valences are based on a particular principle where 1st is valence electrons are the electrons which is the outermost shell of an atom and the 2nd is that it is a noble gas, that does not create any chemical bond.
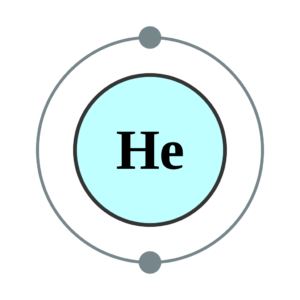
Helium Valence Electrons Dot Diagram
The Lewis symbol is the symbol of Helium. Helium comes in one of the noble gases and has a full valence shell. Helium contains 2 valence electrons. Under the Lewis symbol of helium, where the electrons depict in two lone pair dots. Here is the diagram for the He valence electrons which describe with the help of its diagram. This table will help you learning and understand the whole Lewis symbol electrons dot diagram.
Valency of Helium
Valency is basically that the number of electrons that an atom can gain, lose or share. The children can learn the whole concept with the details given here about the helium. As we know the atomic number of helium is 2 and its electronic configuration is k-2. Here, the maximum number of electrons k shell can accommodate which is 2. Which means that its duplet is completed. So, helium does not have to gain or lose an electron. And due to which valency of helium will be 0.

Leave a Reply