Cobalt Electron Configuration: Chemical element Cobalt has a 27th atomic number and its symbol is “Co”. Cobalt exists on the earth’s crust and it is in chemically combined form, its presence in the form of small deposits of natural meteoroid iron alloys. It is silver-grey metal or a hard, luster-free element. It is an inner transition element that is present in the 4th group of the periodic table.
- Sodium Electron Configuration
- Germanium Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
Cobalt Electron Configuration
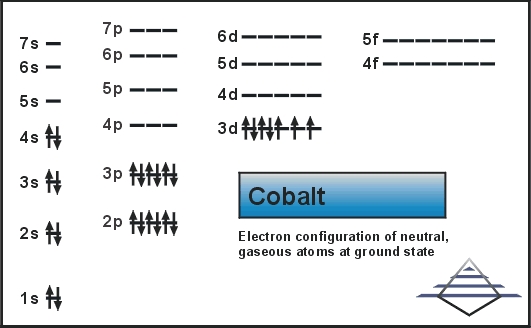
The number of electrons in the orbits of atoms and molecules is termed as electronic configuration. In this case of Cobalt there are 27 electrons which are present in 4 orbits and their distribution on the orbit that is electronic configuration can be written as:
1s22s22p63s23p63d74s2.
- Fluorine Electron Configuration
- Neon Electron Configuration
- Vanadium Electron Configuration
- Clorine Electron Configuration
- Clorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Technetium Valence Electrons
- Vanadium Valence Electrons
- Manganese Valence Electrons
- Cobalt Valence Electrons
- Copper Valence Electrons
- Gallium Valence Electrons
- Arsenic Valence Electrons
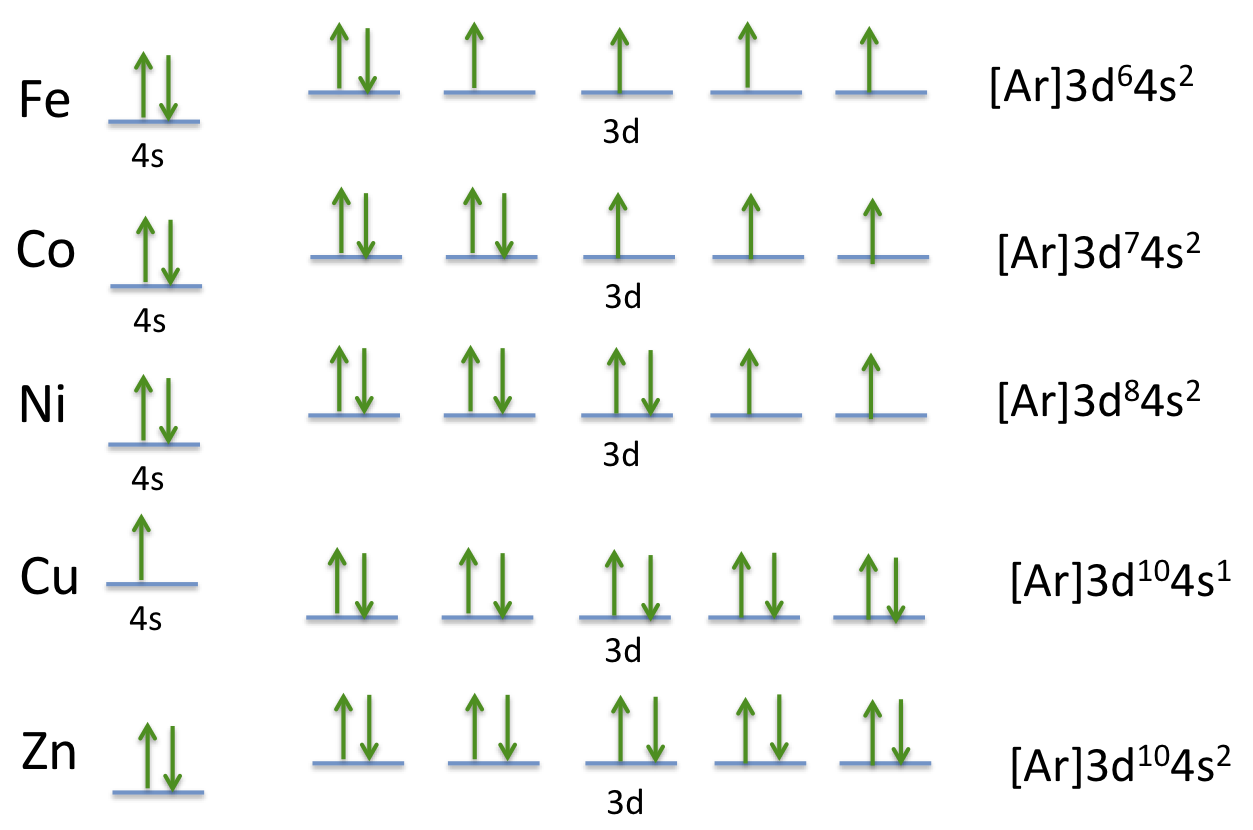
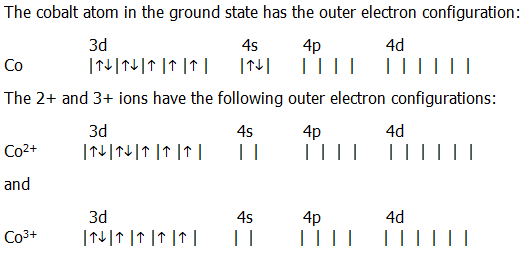
Electron Configuration For Cobalt ion
Electron distribution of electrons in atom or molecules is defined as electron configuration and electron configuration for cobalt ion can be represented as:
1s22s22p63s23p63d74s2. Or [Ar]3d74s2.
Full Electron Configuration For Cobalt
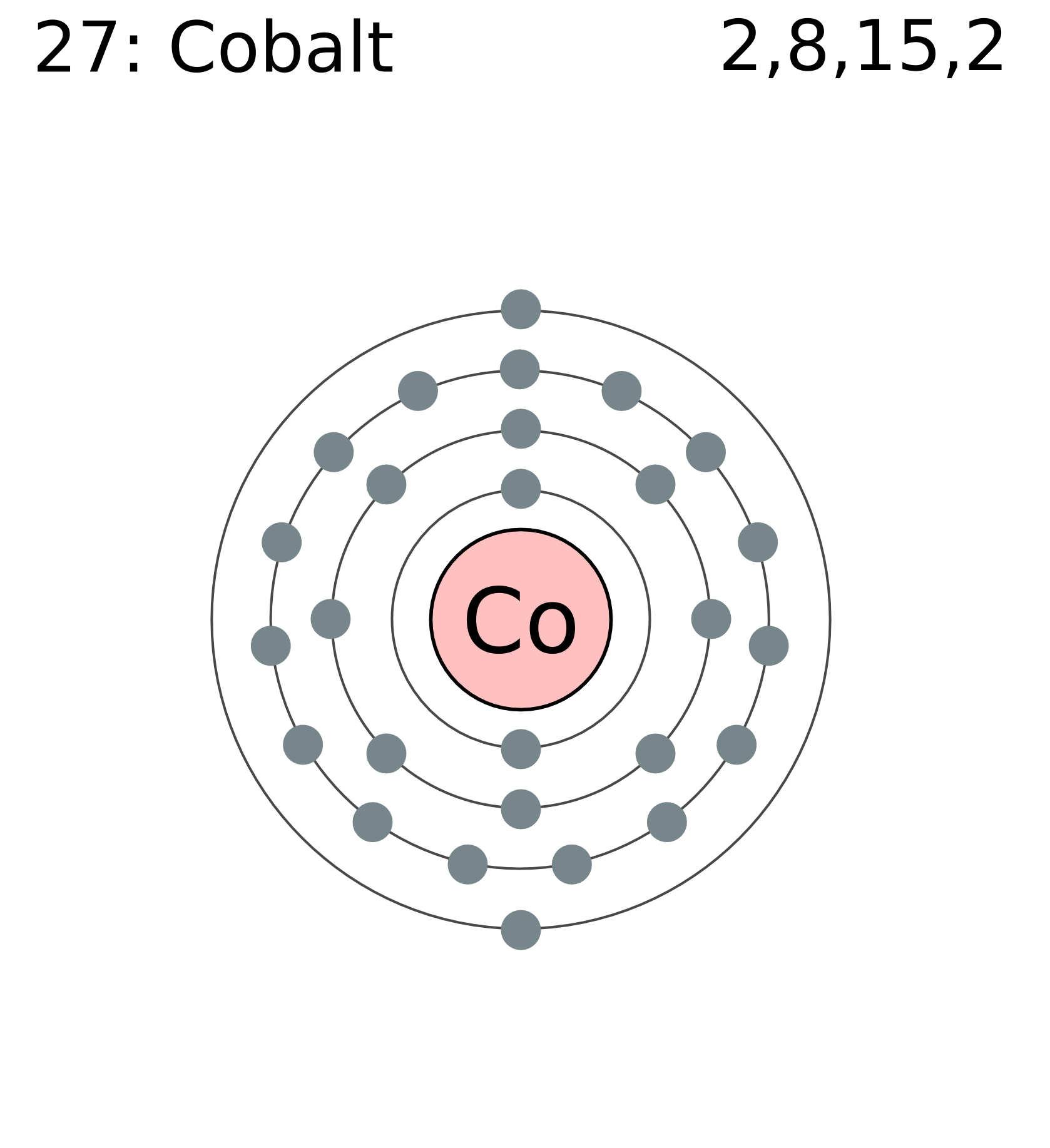
Full electron configuration can be defined as 27 electrons distribution in 4 shells of Co element. There are 2, 8, 15, 2 elements present in the 4 orbits of cobalt and can be represented in this form: 1s22s22p63s23p63d74s2.
What is The Electron Configuration of Cobalt
Electron distribution of cobalt in s, p, d blocks of 4 orbits is 2, 8, 15, 2 and it can be written as: 1s22s22p63s23p63d74s2.
How Many Valence Electrons are in Cobalt
Valence electrons are e– (electrons) of the atom present in the outermost shell. And in the case of cobalt, there are 8 valence electrons.
Leave a Reply