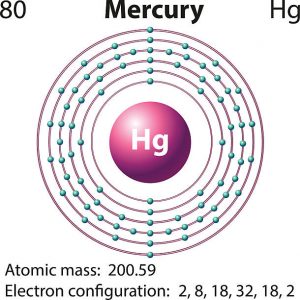
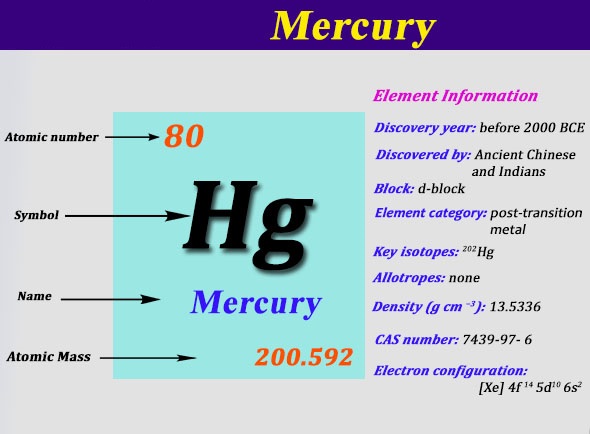
Mercury is a chemical element which has a chemical symbol Hg. The atomic number of mercury is 80. It is popularly known as Quicksilver and was previously named as hydrargyrum. A silvery d-block, heavy element, mercury is the only metal which is liquid at favourable conditions for pressure and temperature; the only other metallic element which is liquid under such conditions is bromine, though metals such as gallium, caesium, and rubidium that melt just above room temperature.
- Sodium Electron Configuration
- Germanium Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
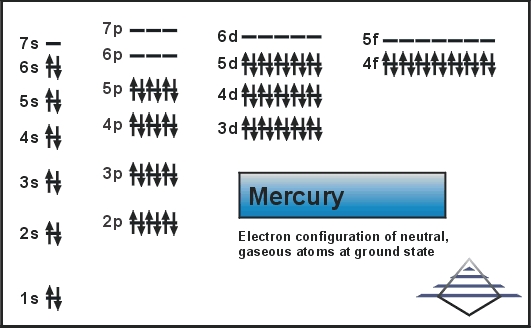
- Mercury Electron Configuration
Mercury is found in deposits throughout the world mostly as mercuric sulfide ( cinnabar). The red coloured pigment vermilion is obtained by grinding natural synthetic mercuric sulfide or cinnabar. It is used in barometers, thermometers, sphygmomanometers, manometers, mercury switches, float valves, fluorescent lamps mercury relays, and other devices, though concerns about the mercury’s toxicity have led to mercury sphygmomanometers and thermometers as largely phased out in clinical areas in favour of alternatives such as Galinstan or alcohol- filled glass thermometers and infrared-based electronic instruments or thermistor.
Similarly, electronic strain gauge sensors and mechanical pressure gauges have replaced mercury sphygmomanometers. Today we are here to share the information about the electron configuration of mercury. Please have a look at the full article for more information.
What is the Electron Configuration of Mercury?
[Xe] 4f14 5d10 6s2 is the electron configuration for mercury.
How Many Valence Electrons Does Mercury have
Mercury has two valence electrons in its outer shell.
Mercury Number of Valence Electrons
There are two valence electrons in the outer shell of the mercury.
Leave a Reply