Magnesium Electron Configuration: Mg is a chemical element that has the symbol Mg. The atomic number of Magnesium is 12. It is a grey shiny solid that bears a close physical resemblance to five other elements of the second column and group 2 (alkaline earth metals) of the periodic table.
Magnesium Electron Configuration
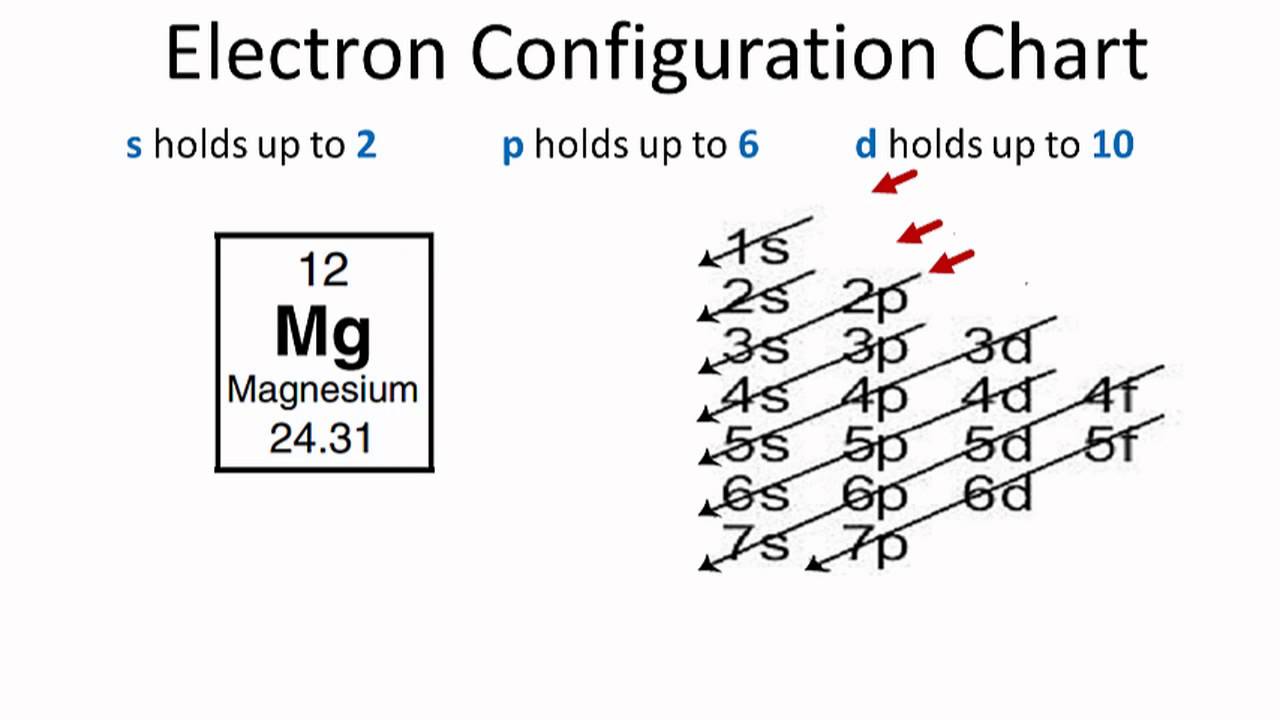
All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The ninth most abundant element in the universe. Today we will tell you about the electron configuration of the Mg.
What is the Electron Configuration of Magnesium
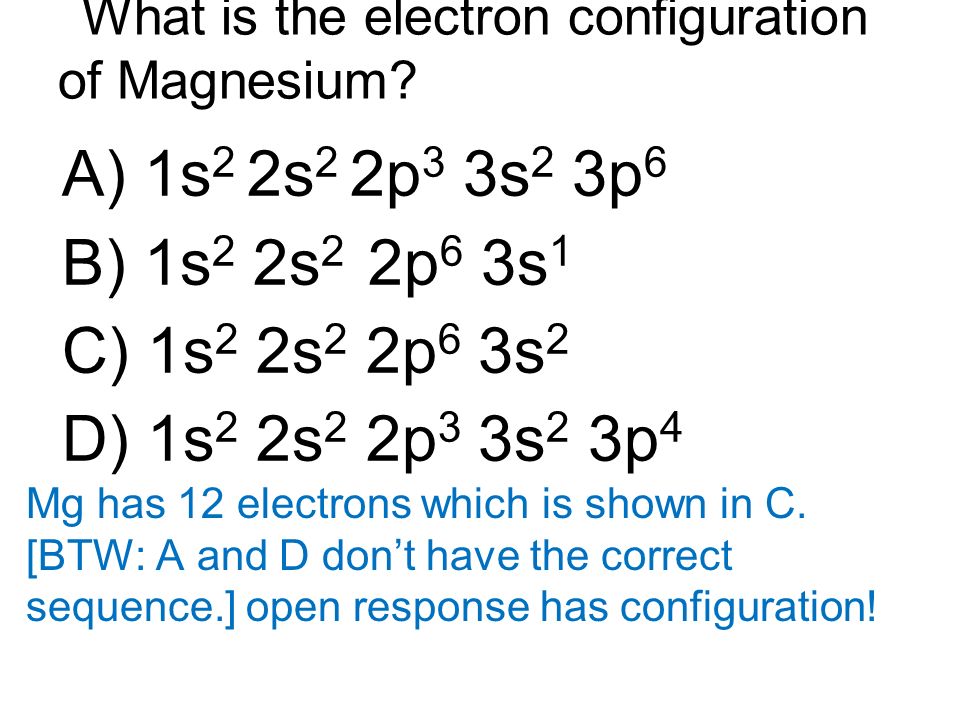
Ne 3s2 is the electron configuration for Mg. Many other electrons configurations of other elements have been provided here for the user. You can check it here:
- Ar Valence Electrons
- K Valence Electrons
- Ca Valence Electrons
- Ne Electron Configuration
- Vanadium Electron Configuration
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- Hydrogen Electron Configuration
- Helium Electron Configuration
- Lithium Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- Carbon Electron Configuration
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Aluminum Valency
- Silicon Valency
- Phosphorus Valency
- Sulfur Valency
- Clorine Valency
- Argon Valency
- Potassium Valency
- Calcium Valency
- Titanium Valency
- Vanadium Valency
- Chromium Valency
- Manganese Valency
- Cobalt Valency
- Nickel Valency
How Many Valence Electrons Does Magnesium Have
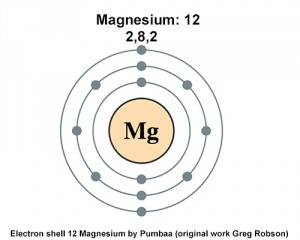
Magnesium has two valence electrons in its outer shell. As you can see in the given picture which is provided below.
Magnesium Number of Valence Electrons
There are two valence electrons in the outer shell of the Magnesium.
Ground State Electron Configuration of Mg
1s2 2s2 2p6 3s2 is the Ground-state Electron Configuration of the Mg.
Many other valence electrons of the element have been available here: