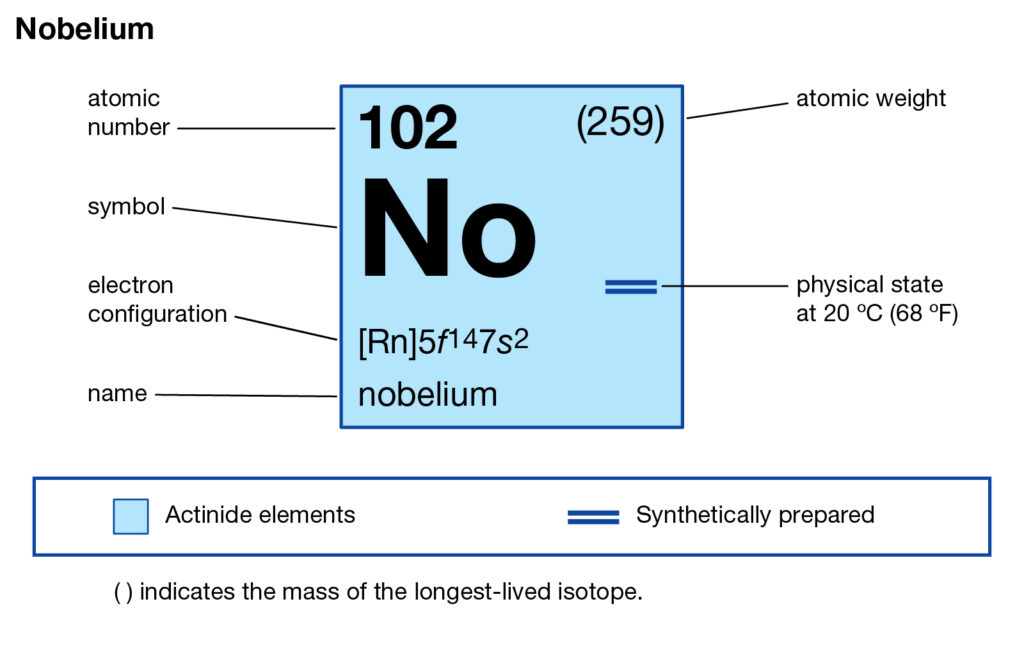
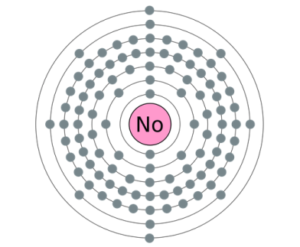
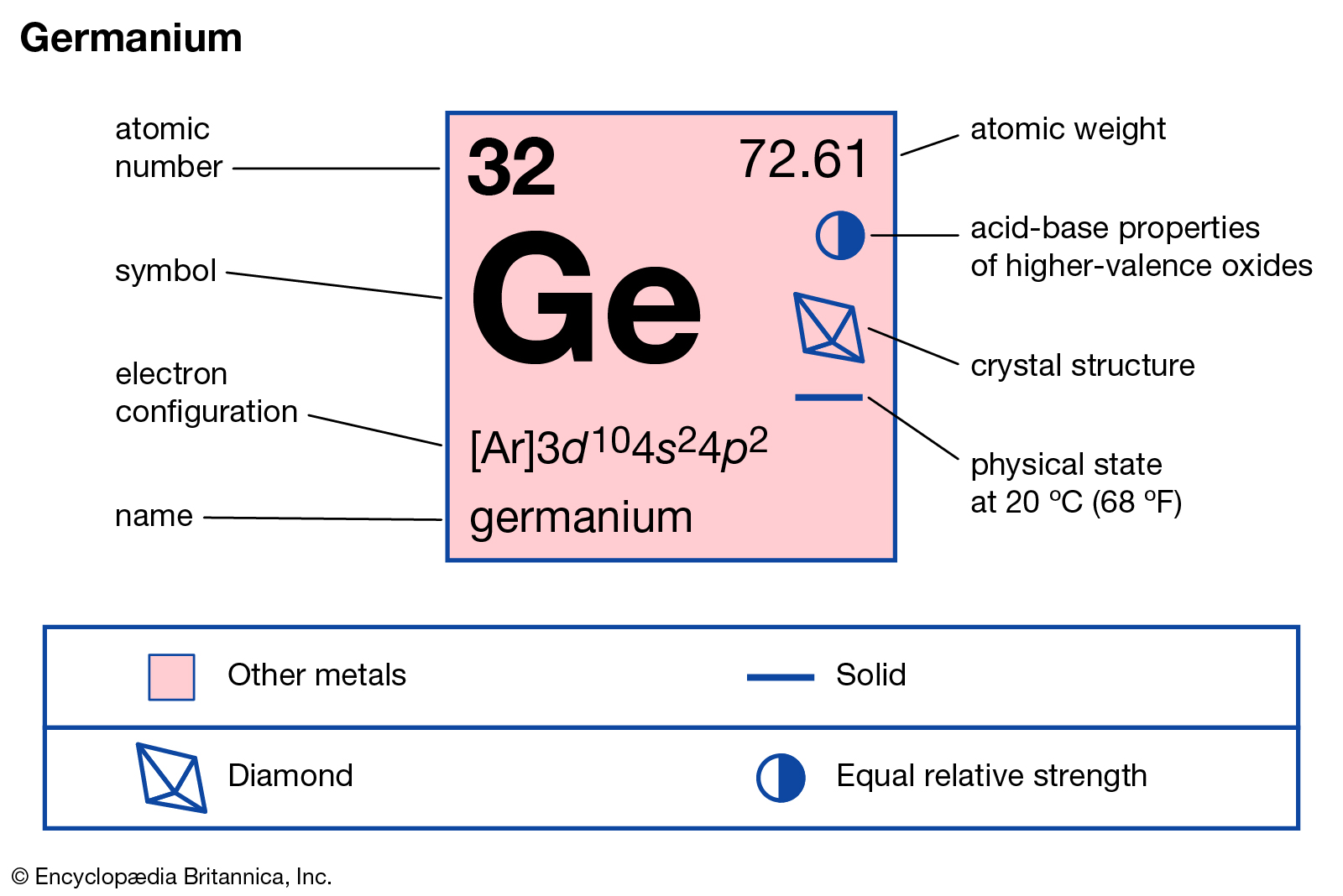
Readers can here study the Nobelium valence electrons to enhance their chemistry knowledge. We further have other useful information about the element. Nobelium is a pure chemical element in the language of chemistry. It has the atomic number as 102 and the symbol of No.
How many valence electrons does Nobelium have?
Nobelium is one of those chemicals elements which have no natural existence. It is a purely synthetic chemical element since its human-made. Nobelium is made by the particle accelerator process by scientists. In this process, the charged particles rapidly bombard the lighter elements.
So, this is how with this process Nobelium takes birth. There is a variety of Nobelium, but some of them are stable while others are highly unstable.
So, in short, Nobelium is a lab-made highly reactive chemical element. The element is only useful for some research purposes. It has no as such biological usages either in any of the proven field. The element is still in its pure research phase to figure out its useful usage.
Nobelium Valence Electrons Dot Diagram
The Lewis dot diagram is probably the best source of learning about the valence electrons of Nobelium. With this dot diagram, you can explore the chemical bonding of valence electrons of atoms.
Furthermore, the diagram will also let you know about the bonding pattern of valence electrons. The single pair of dots convey the single bonding while the double pair of dots means double bonding.
Valency of Nobelium
Well, Nobelium can hold 4 valence electrons in its outer shell. The valency of Nobelium is hence exactly 4 in its oxidation state.
Valency of Nobelium is the combining capacity of this element. So, No may gain or lose 4 valence electrons to attain stability.