Tin Electron Configuration: Tin is a chemical element that has the symbol Sn (It is taken from the Latin word tannum). The atomic number of Tin is 50. It is one of the post-transition metals in group 14 of the periodic table. It is obtained mainly from the mineral cassiterite that contains stannic oxide, SnO2.
- Sodium Electron Configuration
- Germanium Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
Tin Electron Configuration
-
Element Symbol: Sn
-
Atomic Number: 50
-
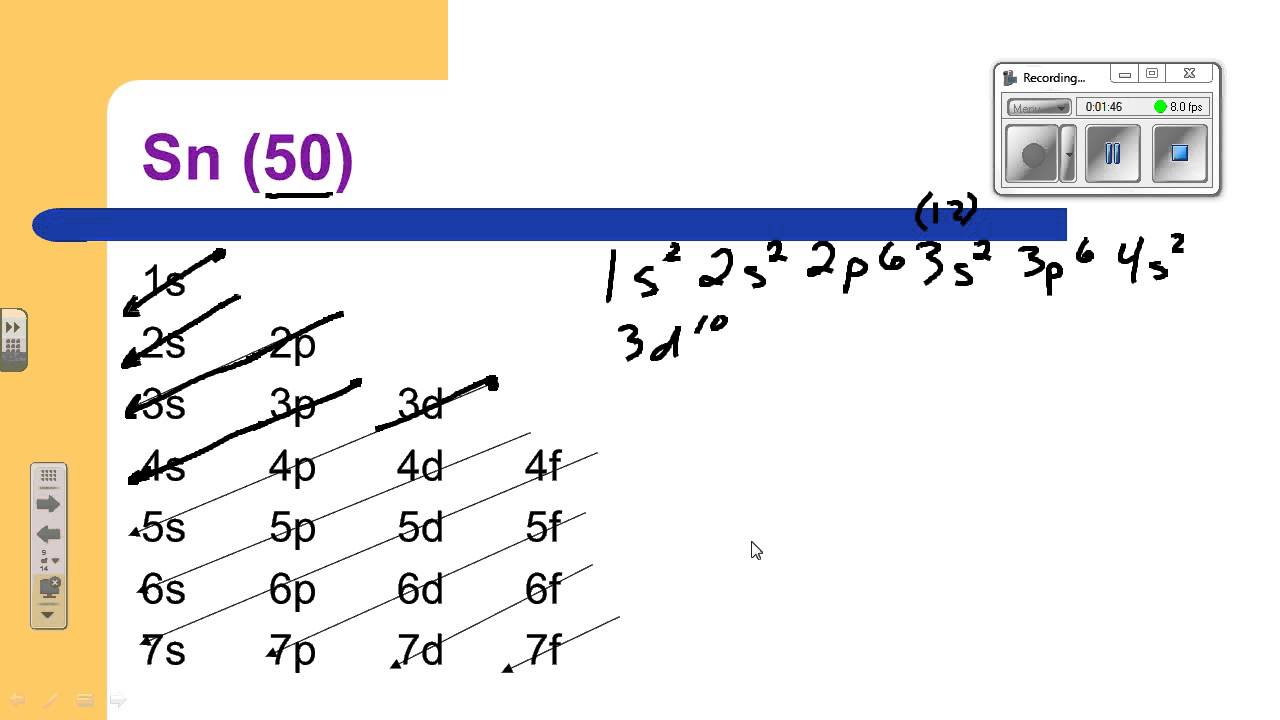
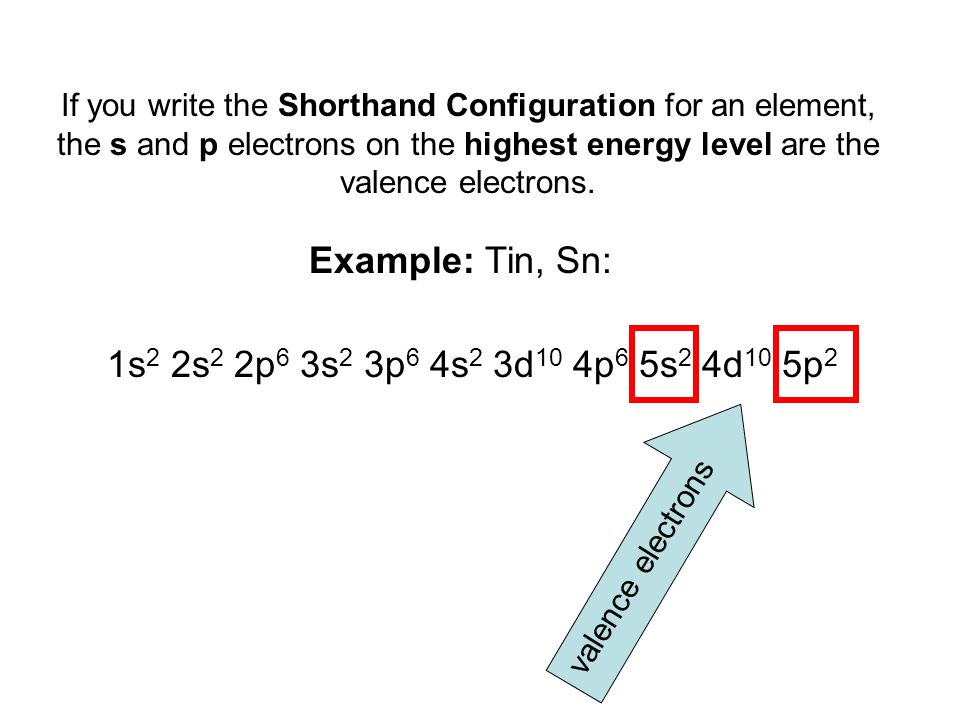
Full Configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p²
-
Shorthand Configuration: [Kr] 4d¹⁰ 5s² 5p²
-
Valence Electrons: 4 (from 5s² 5p²)
-
Group: 14 (IVA), Period: 5
-
Common Oxidation States: +2, +4
It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4. It is the 49th most abundant element which has 10 stable isotopes which is the largest number of stable isotopes in the periodic table because of its magic number of protons.
Tin has two major allotropes: at room temperature, the stable allotrope is β-tin, malleable metal, a silvery-white. At low temperatures, it gets transformed into the less dense grey α-tin, that has the diamond cubic structure. Metallic tin does not get easily oxidized in air.
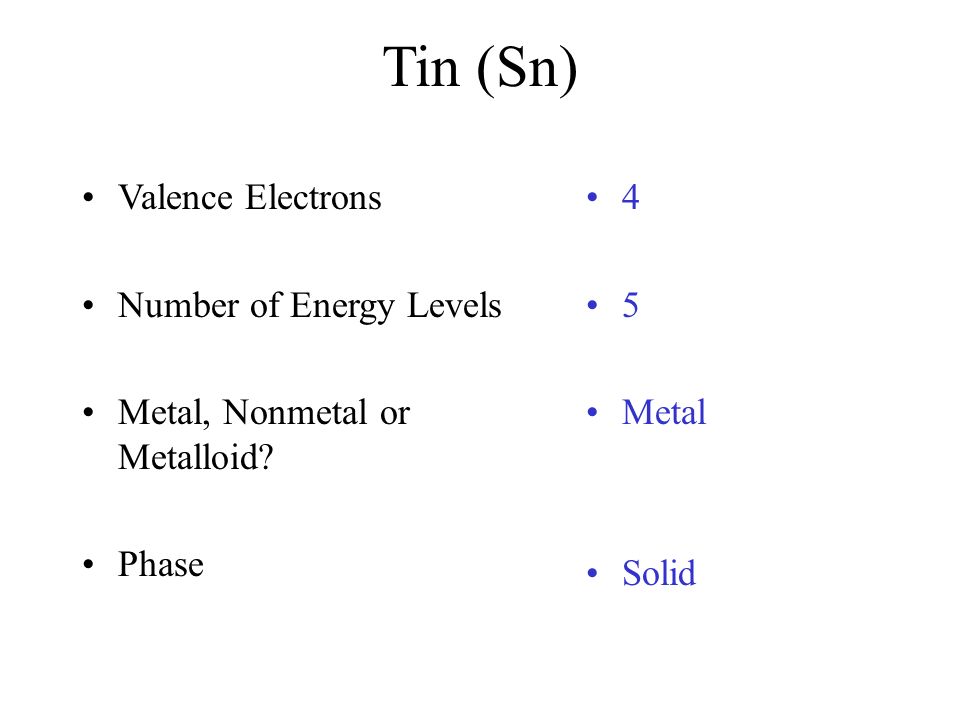
Tin number of Valence Electrons
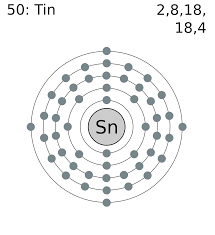
The Tin has 4 valence electrons in its outer shell.
Valence Electrons of Tin
See the picture below to know the valence electron of the Tin.
What is The Electron Configuration of Tin
The full Electron Configuration of Tin is 1s 22s 22p 63s 23p 64s 23d 104p 65s 24d 105p 2
How Many Valence Electrons Does Tin Have
The total number of the valence electron in the outer shell of tin is 4
Leave a Reply