You can here read everything about the Copper valence electrons for your knowledge. We shall also discuss and describe the other characteristics of this element in the article. Copper is one of the most commonly known chemical elements in chemistry. The element has significant popularity due to its wide usages.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Sulfur Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
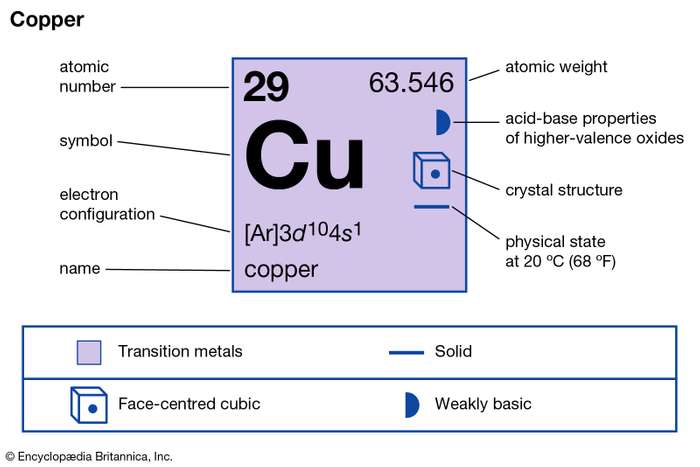
How many valence electrons does Copper have?
Copper has the atomic number 29 and the representative symbol as Cu. In composition, copper has the structure as a soft, malleable metal. The color of freshly exposed copper generally remains as orange in color. Copper is one of the most highly conductive medium of electricity.
Copper remains in very high demand and usage due to its high electrical conductivity. Most of the electrical wires and components have the coppers within them.
Copper has its directly occurring form in nature in many countries around the world. Furthermore, the copper has the numerous usages since the very ancient time in history. Moreover, copper is even an integral part of the human bodies. It’s the significant part of human organs as the respiratory enzymes.
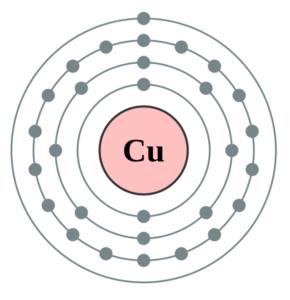
Copper Valence Electron Dot Diagram
You can understand the valence electrons with better insight by the dot diagram. We call the dot diagram as Lewis dot diagram for valence electrons of elements. The dot diagram simply represents the numbers of valence electrons of atoms. It draws the dots around the symbol of copper to show up the valence electrons.
The numbers of dots remain equal to the numbers of valence electrons of atoms. Lewis dot diagram can help you in the proper analysis of copper valence electrons.
Valency of Copper
The valency of copper in the general scenario remains either +1 or +2. The reason being copper is a transition metal hence it doesn’t have the obvious valency.
The metal elements like copper don’t form covalent bonds. The valency of these elements hence remains on the corresponding compounds.