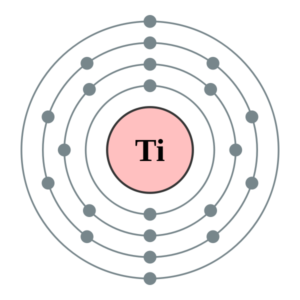
Get to understand the Titanium Valence electrons here in our article. You can also understand the other key characteristics of this element in the article. Titanium in chemistry is known as the chemical element. It has the atomic number 22 and symbol as Ti.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Sulfur Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
How many valence electrons does Titanium have?
Titanium is undoubtedly one of the strongest chemical elements in the world. It is a pure metal with a solid grey composition and low density. The history of Titanium comes from Greek mythology back in the year 1791. In the present scenario, Titanium has become a highly useful metal.
Titanium is the key component of several industries around us in their manufacturing process. Titanium is easily allowable with other metals such as iron, steel, etc. You can witness the application of titanium even in the production of spacecraft. In conclusion, this chemical element is the key element of the metal industry.
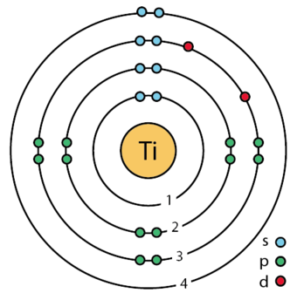
Titanium Valence Electrons Dot Diagram
Lewis dot diagram is the best tool for the valence electrons representation of atoms within the molecule. The diagram is relevant in visualizing the valence electrons of atoms and molecules together.
The diagram basically uses the dots to represent the electrons in the outer shell of the atom. With the help of this diagram, you can figure out the single or dual bonding of atoms and molecules.
Valency of Titanium
The valency of titanium is accurately four to attain stability. Valency is basically the integral properties of Titanium just like the other chemical element.
The four valencies of Titanium states that it may either gain or lose 4 electrons. The outermost shell of Titanium holds the s and p electrons of this element. We further urge you to read the electron configuration of Titanium. It will guide you in concluding the valency of Titanium.