Electron configuration can be defined as the number of electrons or distribution of electrons of the molecules or atoms in orbit. Lead is the chemical element which has atomic number 82 AND symbol “Pb”. This chemical element is most common and heavy metal.
- Hydrogen Electron Configuration
- Silicon Electron Configuration
- Helium Electron Configuration
- Lithium Electron Configuration
- Aluminum Electron Configuration
- Beryllium Electron Configuration
- Boron Electron Configuration
- Scandium Electron Configuration
- Sulfur Electron Configuration
- Sodium Electron Configuration
- Germanium Electron Configuration
- Carbon Electron Configuration
- Nitrogen Electron Configuration
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
This metal has a low melting point and soft and malleable. This is easy to cut. This metal is silvery and slightly blue but turns to be dull grey when came in contact of air.

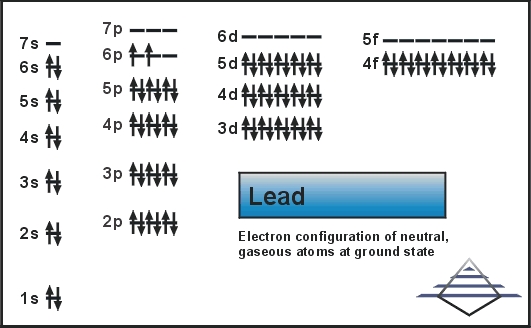
This element has 82 number of electrons present in its 6 orbits. The distribution of electrons is 2, 8, 18, 32, 18, and 4.
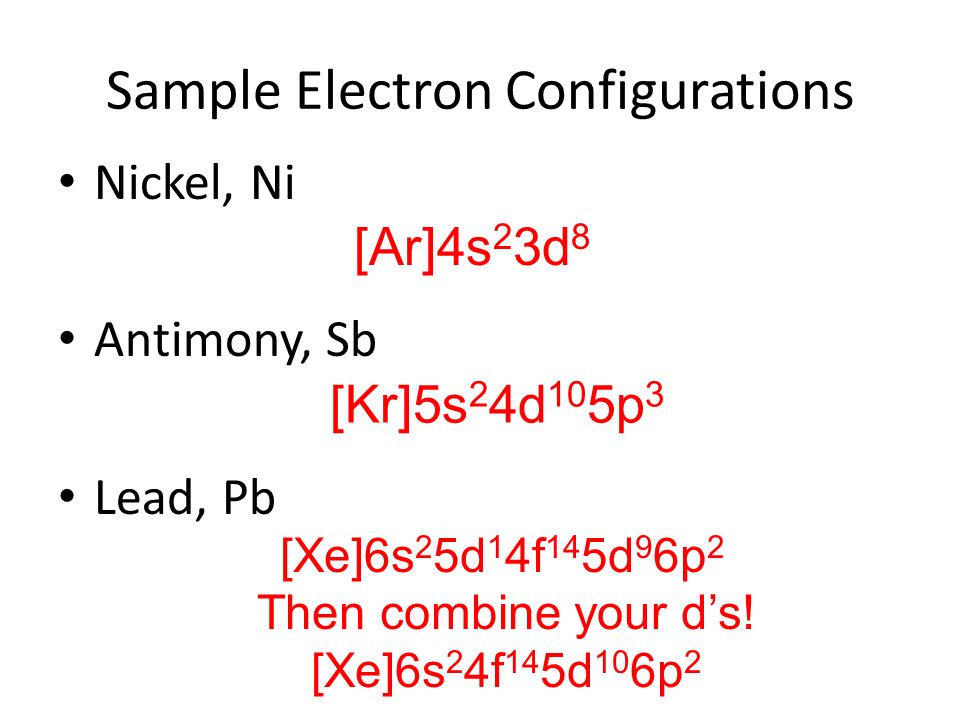
Electronic configuration for lead can be written as: 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p2
Or
[Xe] 4f145d106s26p2
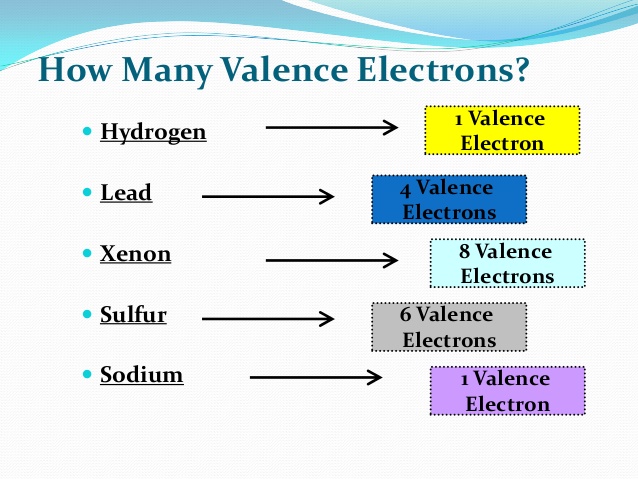
Lead Valence Electrons
Valence electrons are electrons in the outer most shell or orbit and there are 4 valence electrons present in the lead (Pb) outermost shell.
Full Electron Configuration For Lead
Full electron configuration for lead is 1s22s22p63s23p63d104s24p64d104f145s25p65d106s1.
Or it can also be written as
[Xe] 4f145d106s1
What is The Electron Configuration of Lead
Electrons distribution in the orbit of atom or molecule is termed as electron configuration. And electron configuration of Pb (lead) is defined as 1s22s22p63s23p63d104s24p64d104f145s25p65d106s1.
How Many Valence Electrons Does Lead have
A number of valence electrons are defined as a number of electrons present in the outer shell of atom or molecule. In case of lead, there are 4 electrons present in the 6th or outer shell. So, a number of valence electrons in lead is 4.
Leave a Reply