Sulfur Electron Configuration: Sulphur or sulfur is a chemical element. It has a chemical symbol S. The atomic number of Sulfur is 16. It is multivalent, abundant, and nonmetallic. Under normal situations, sulfur forms cyclic octatomic molecules that have a chemical formula S8.
Sulfur Electron Configuration
Elemental sulfur is a yellow bright crystalline solid at room temperature. Sulfur chemically reacts with all elements except for platinum, gold, tellurium, iridium, and the noble gases. It is the fifth most common element by mass on Earth and tenth in the universe.
It is sometimes found in the pure and native form. Sulfur usually occurs as sulfate and sulfide minerals. As it is abundant in native form, sulfur was also known in ancient times. It was mentioned for its uses in ancient Greece, ancient India, Egypt and China.
- Chlorine Valence Electrons
- Argon Valence Electrons
- Potassium Valence Electrons
- Calcium Valence Electrons
- Technetium Valence Electrons
- Titanium Valence Electrons
- Vanadium Valence Electrons
- Chromium Valence Electrons
- Manganese Valence Electrons
- iron Valence Electrons
- Cobalt Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
- Zinc Valence Electrons
- Gallium Valence Electrons
- Germanium Valence Electrons
- Arsenic Valence Electrons
- selenium Valence Electrons
In the Bible, sulfur is known by the name of brimstone. Now all elemental sulfur is made as a byproduct of removing sulfur-containing contaminants from petroleum and natural gas. Today we are going to tell you about the electron configuration of Sulfur.
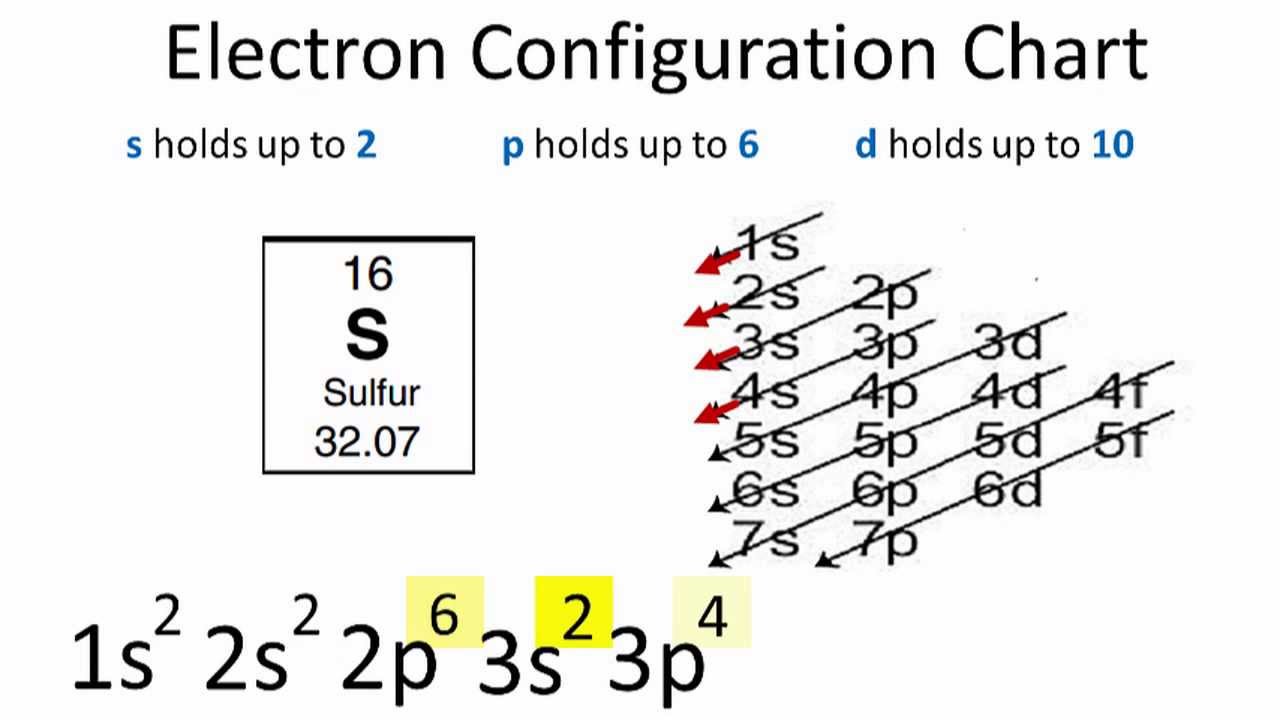
What is The Electron Configuration of Sulfur
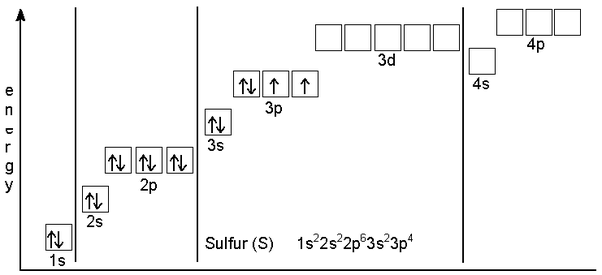
How Many Valence Electrons Does Sulfur Have
Sulfur has six valence electrons in the outer shell of the Sulfur.
Sulfur Number of Valence Electrons
There are six valence electrons in the outer shell of the Sulfur.
Ground State Electron Configuration of Sulfur
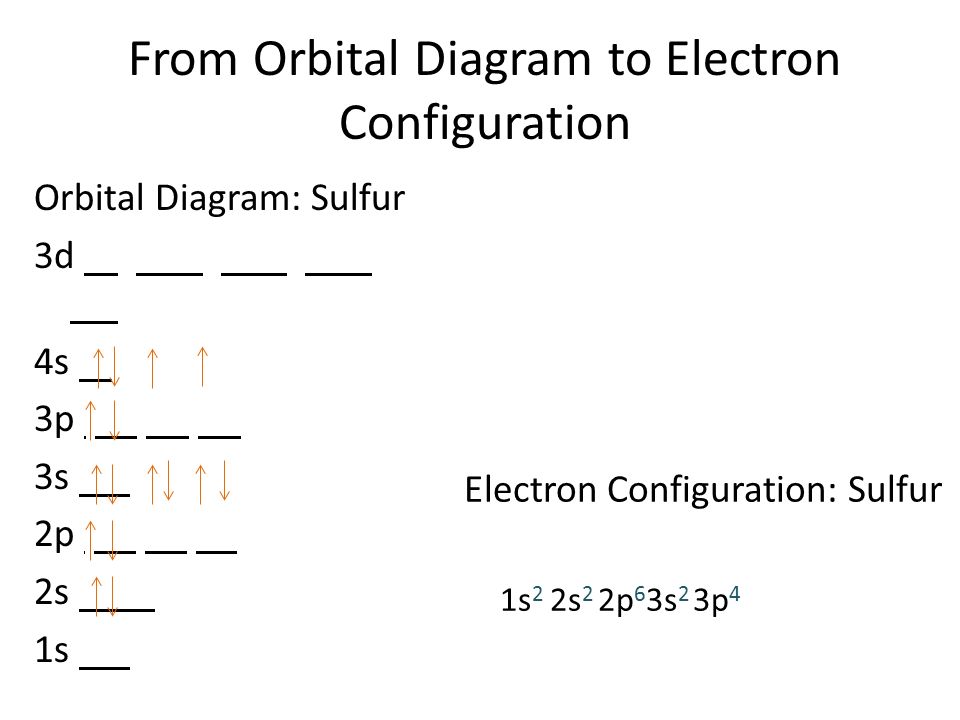
when we the electron configuration of Sulfur the first two electrons go in the 1s orbital. As 1s only hold two electrons and the next two electrons for sulfur goes to the 2s orbital. Hence the S electron configuration is 1s22s22p63s23p4.
Leave a Reply