Scandium Electron Configuration: Scandium is the second lightest chemical element of Halogens. This chemical element has atomic number 21 and symbol “Sc”. This Scandium element lies between Fluorine and Bromine of the periodic table. This Scandium at room temperature is a yellow-green gas. The properties of Scandium are intermediate between elements silvery-white solid.
- Germanium Electron Configuration
- Arsenic Electron Configuration
- Selenium Electron Configuration
- Bromine Electron Configuration
- Krypton Electron Configuration
- Rubidium Electron Configuration
- Strontium Electron Configuration
- Yttrium Electron Configuration
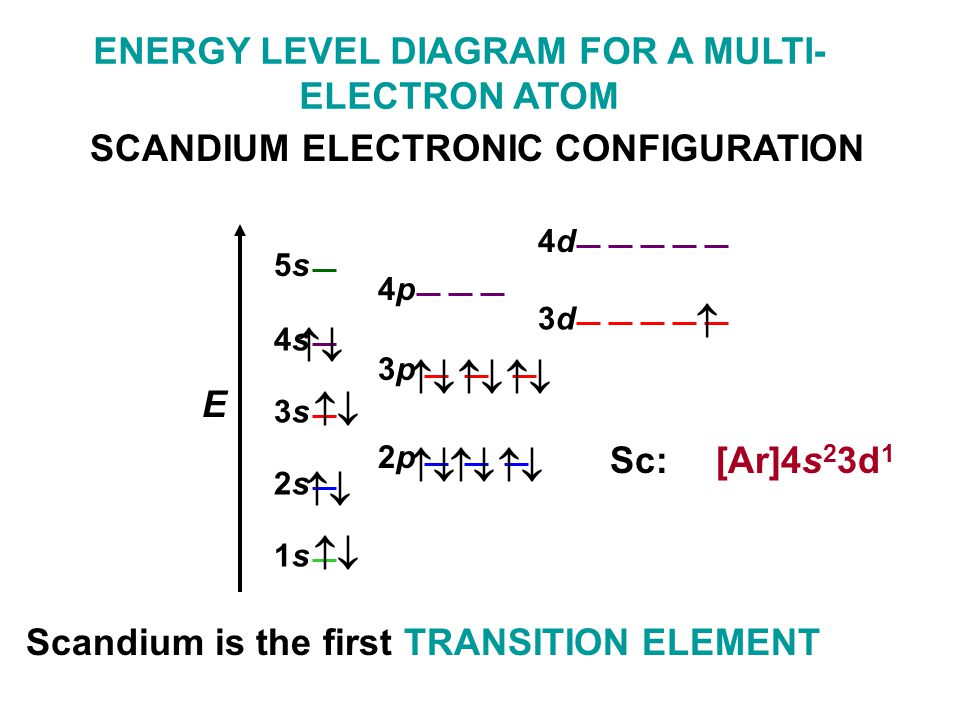
Scandium Electron Configuration
Element configuration for atoms or molecules is defined by the number of electrons present in the orbit or shell. In case of Scandium, there are 3 orbits and 17 electrons present in these orbits or shells. Distribution of electrons in shells is 2, 8, and 7.
Electron configuration of Sc is:
1s2 2s2 2p6 3s2 3p6 3d1 4s2.
Electron Configuration For Scandium ion
Electron configuration of Sc ion can be represented by the distribution of electrons in the shell i.e. 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹ 4s² or [Ar] 3d14s2.
Full Electron Configuration For Sc
Full electron configuration for Sc can be represented as: [Ar] 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹ 4s².
What is The Electron Configuration of Scandium
Electrons which lie in the orbits of atom or molecules is termed as Electronic Configuration. Electronic configuration of Scandium define as: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹ 4s²
How Many Valence Electrons are in Scandium
Valence electrons are a number of electrons lie in the outer most shell. There are 21 electrons which are present in the third or outer most shell i.e. 3d14s2.


Leave a Reply