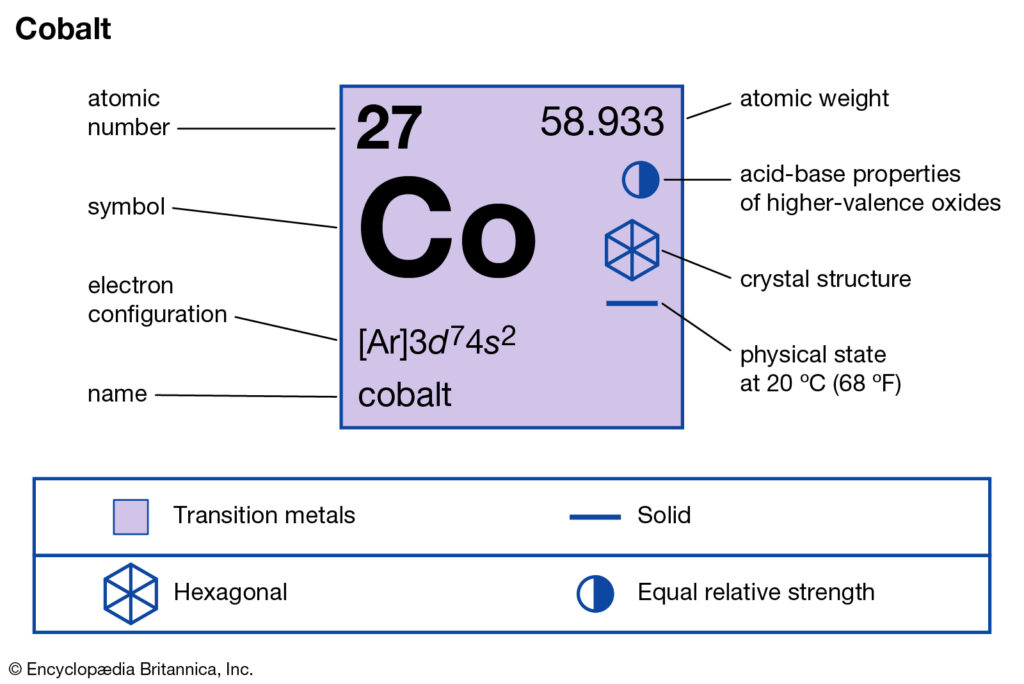
You can here study the Cobalt valence electrons for in-depth knowledge of elements. We would also provide some significant characteristics of this element. In the field of science Cobalt is a highly useful chemical element. It has the representation symbol of Co and the atomic number 27.
- Flerovium Valence Electrons
- Plutonium Valence Electrons
- Mercury Valence electrons
- Neptunium Valence Electrons
- Moscovium Valence Electrons
- Cesium valence electrons
- Bismuth Valence electrons
- Silicon Valence Electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Xenon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Hydrogen Valence Electrons
- Phosphorus Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Sodium Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Aluminum Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Fluorine Valence Electrons
- Magnesium Valence Electrons
- Neon Valence Electrons
- Sulfur Valence Electrons
How many valence electrons does Cobalt have?
Cobalt is extracted from the earth’s crust in the form of combination with other elements. It has the appearance as hard silvery or gray metals. Cobalt has a long history as it first appeared in the use long back during 1735. It was first discovered, as the by-product of nickel and copper.
In the present time, Cobalt has emerged as a highly valuable chemical element. It has wide industrial usage in the production houses.
One of the most important uses of Cobalt is in the production of Lithium-ion batteries. Cobalt is a revolutionary element in giving a boost to electric vehicle production. Cobalt has strong properties in the production of alloys. The alloys made of cobalt are used in aircraft, turbine engines, etc.
Cobalt Valence Electron Dot Diagram
Lewis dot diagram is highly useful in the representation of valence electrons of elements. The diagram shows the numbers of valence electrons of atoms.
It basically uses the dot around the symbol of the given chemical element. Further, the diagram also shows the bonding of atoms and molecules’ valence electrons. You can see the lewis dot diagram of Cobalt just like any other chemical element. It would therefore provide you a picture of Valence electrons for cobalt.
Valency of Cobalt
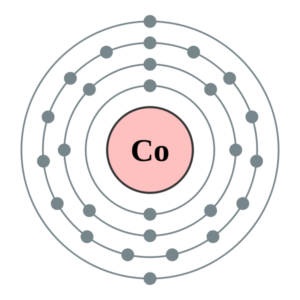
Well, the Cobalt has the exact valency of 1 as its combining capacity. Valency is basically the number of electrons in the outermost shell of Cobalt. So, Cobalt has the electron configuration as 2,8,8,1. It hence proves the number of electrons in its outermost energy shell.
Leave a Reply