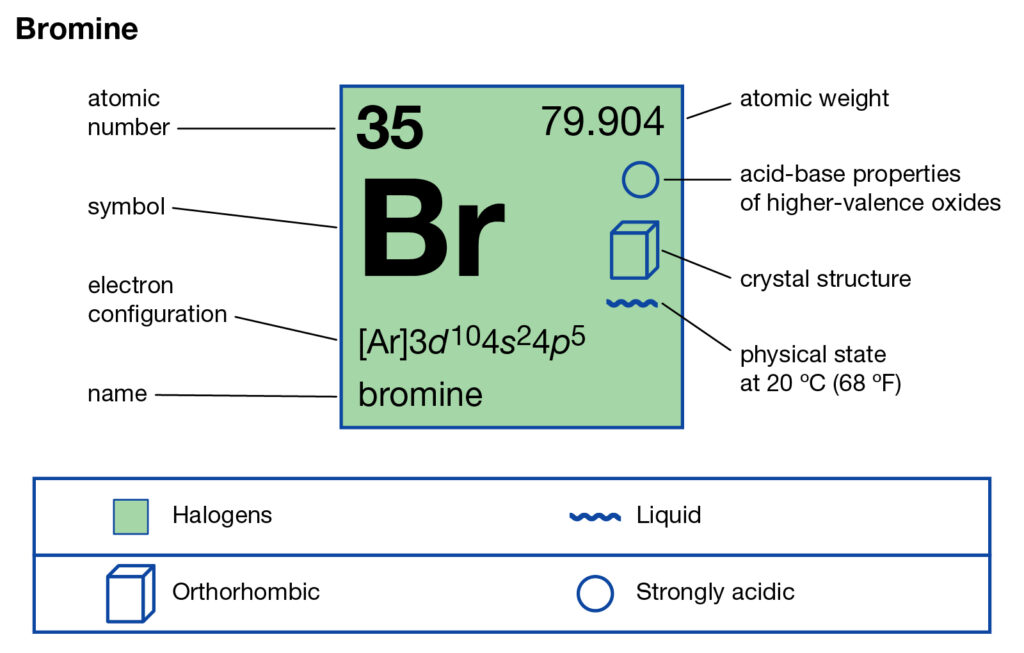
Get a thorough understanding of Bromine valence electrons here in the article. You will further explore the elements with its others significant properties. In chemistry, Bromine is a chemical element with its atomic number 35. It has the writing symbol as Br in the periodic table.
- Flerovium Valence Electrons
- Plutonium Valence Electrons
- Mercury Valence electrons
- Neptunium Valence Electrons
- Moscovium Valence Electrons
- Cesium valence electrons
- Bismuth Valence electrons
- Silicon Valence Electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Xenon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Hydrogen Valence Electrons
- Phosphorus Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Sodium Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Aluminum Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Fluorine Valence Electrons
- Magnesium Valence Electrons
- Neon Valence Electrons
- Sulfur Valence Electrons
How many valence electrons does Bromine have?
Bromine belongs to the group of halogen and hence has similar properties. Being the halogen element Bromine can easily evaporate at a given room temperature. Bromine was first discovered in the year 1825 by two chemists together. It further has the chemical properties between Iodine and Chlorine.
In nature Bromine has the least free form hence it occurs from the mineral water spring. The second source of Bromine lies in the earth’s crustal rocks. Bromide salt contains a minor amount of Bromine as the byproduct. Further, Oceans have a significant amount of Bromine but it’s hard for extraction purposes.
United States and Israel are the countries to have the significant native source of Bromine. The countries generally export the required amount of Bromine to other nations.
The major consumption of Bromine goes to the production of Organobromine. The other significant part is useful in the production of flame retardants. Some amount of Bromine is useful to use as the pesticides on the crops. So, in conclusion, Bromine has very limited practical applications as of now.
Bromine Valence Electrons Dot Diagram
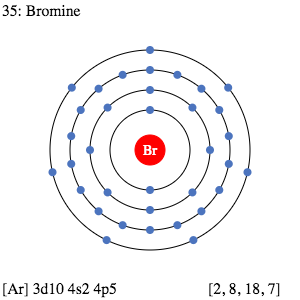
You can study the valence electrons of Bromine with the help of the Lewis dot diagram. The diagram breaks down the total numbers of Bromine valence electrons of atoms.
Dot diagram further helps in understanding the interaction of valence electrons. It draws the numbers of dots around the symbol of Br as the valence electrons.
Valency of Bromine
Well, the exact valency of bromine remains 7 as its combining capacity. So,It has the 7 valence electrons in its outermost energy shell.
Leave a Reply