Get to know everything about the Zinc valence electrons here in the article. We shall break out the key information of the element for readers. Zinc is a well-recognized chemical element in chemistry. It has the atomic number as 30 and the representation symbol as Zn.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Sulfur Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
How many valence electrons does Zinc have?
The chemical element looks like a brittle sluggish grey and silver in its general composition. Zinc further closely resembles to Magnesium in its characteristics.
Zinc is commonly found in the earth’s crust just like the other minerals. It’s significantly available in nature for its quite possible usages around. The largest quality of zinc is available in Asia, Australia and the United States. Zinc further has the numbers of usages across the manufacturer’s industries.
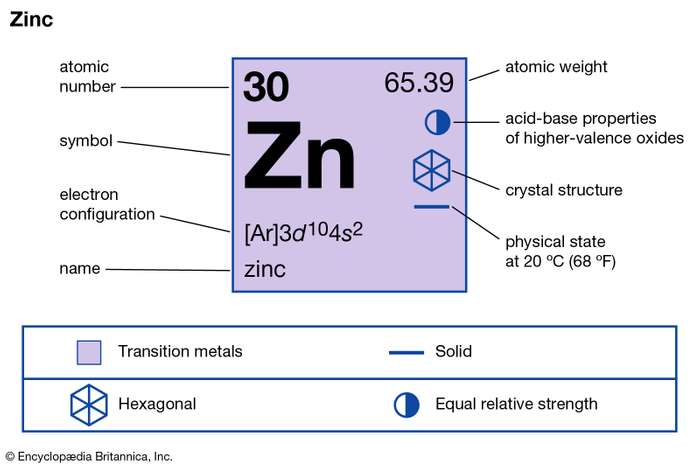
Zinc in its early form has the most common usage in integration with brass and bronze. It also has the anti corrosion properties. Zinc is the common part of the human body just like the other minerals. The deficiency of zinc can cause several kinds of illnesses within the human body.
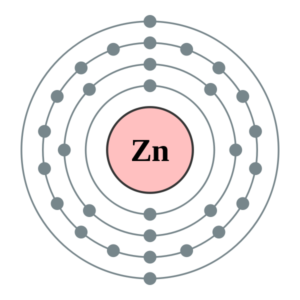
Zinc Valence Electrons Dot Diagram
Readers can understand the valence electrons of zinc by referring to the dot diagram. The Lewis dot diagram explains and draws the numbers of valence electrons of Atom. The dot diagram shows the numbers of dots in equivalent to the numbers of valence electrons. The diagram draws the dots around the symbol of Zinc.
The diagram further helps in understanding the bonding pattern of valence electrons. Single pair of dots represents the single bond, while double pair of dots represents the dual bonding.
Valency of Zinc
The precise and accurate valency of Zinc is +2. Zinc is the metal transition element and hence it belongs to the d block category. The outermost shell of zinc contains 2 electrons hence zinc can lose 2 electrons. So, Zinc gains stability after losing out the 2 electrons.
Leave a Reply