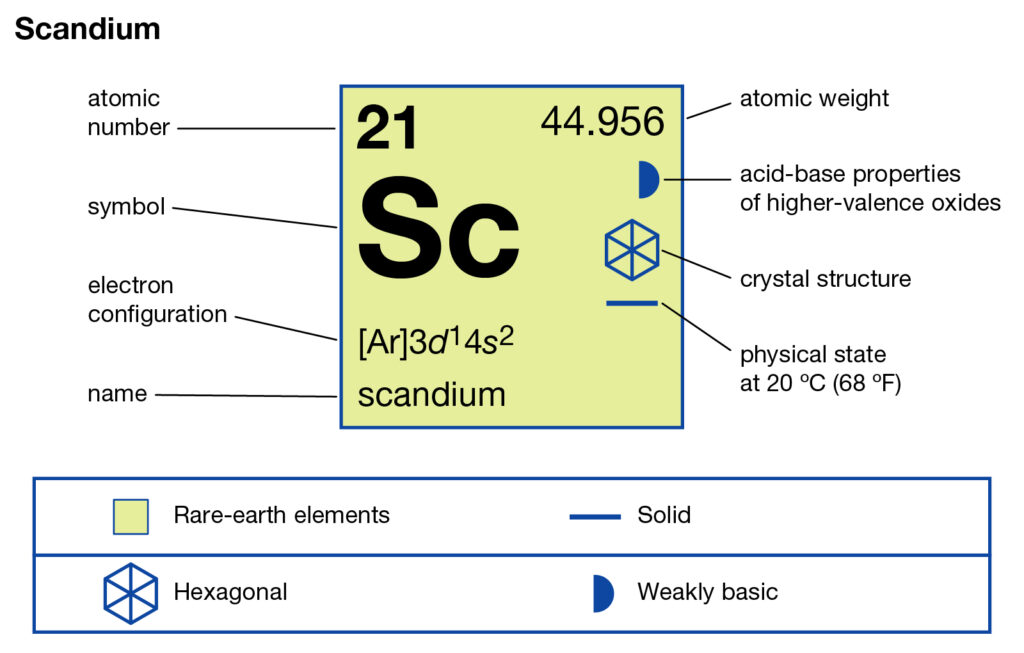
Study the Scandium valence electrons here to know the element in a better way. You can also study the other characteristics and properties of elements in the article. Scandium is basically a d block element in the domain of chemistry. It comes with its atomic number 21 and symbol sc.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Sulfur Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
How many valence electrons does Scandium have?
It’s a rare-earth compound due to its very limited availability within nature. It was first discovered in the year 1879 by the specter analysis process. Scandium is extracted from Uranium and other rare earth compounds. You can consider it as the byproduct of these elements since it has no free form.
The chemical element has properties similar to Aluminum and Ytriuum. Scandium has no radioactive or toxic properties hence it’s nonhazardous for human use. As of now, scandium has no major application in the commercial domain. The limited usage of the element includes in the alloy production.
- Hydrogen Valence Electrons
- Helium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Fluorine Valence Electrons
- Sodium Valence Electrons
- Aluminum Valence Electrons
Scandium basically renders ultimate strength to the alloys and alloys made components. This is why the major aircraft components use alloy and scandium made parts. Scandium generally carries high cost due to its less availability. The element is still im research purpose for its further proven usages.
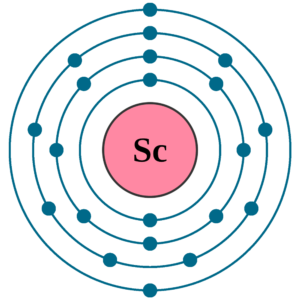
Scandium Valence Electrons Dot Diagram
Users can take a look at the Lewis dot diagram to understand the Sc valence electrons. The diagram helps in understanding the interaction of valence electrons.
With this diagram, you can further figure out the numbers of scandium valence electrons. So, feel free to use the dot diagram to explore more of Scandium valence electrons.
Valency of Scandium
Scandium has a maximum valency of 3 in its oxidation states. You can understand the valency as the combining capacity of Scandium. Valency guides in understanding the numbers of atoms that will combine with other elements. Consequently, the numbers of atoms form a chemical formula.