Molybdenum Electron Configuration: In the present scenario where the newer chemical elements are being discovered on a very rapid pace, it is being more essential to examine those chemical elements. Electron Configuration is the only way to understand and simplify the chemical reaction of these elements. Today in this article we are going to discuss the Electron Configuration of the Molybdenum.
- Bromine Electron Configuration
- Sodium Electron Configuration
- Magnesium Electron Configuration
- Aluminum Electron Configuration
- Silicon Electron Configuration
- Sulfur Electron Configuration
- Chlorine Electron Configuration
- Phosphorus Electron Configuration
- Argon Electron Configuration
- Potassium Electron Configuration
- Scandium Electron Configuration
Molybdenum Electron Configuration
Molybdenum is a chemical element that is not found in the natural form on the earth as it is generated from the oxidation states of the minerals. This chemical element is used as the hard and stable carbides in the alloy.
The Electron Configuration of the Molybdenum implies the distribution of the Molybdenum’s electrons in the atomic molecules. This electronic configuration of Molybdenum helps the chemist, and another scientist to understand the chemical reaction of the Molybdenum so that it could be used for suitable purposes.
Electron Configuration of Molybdenum?
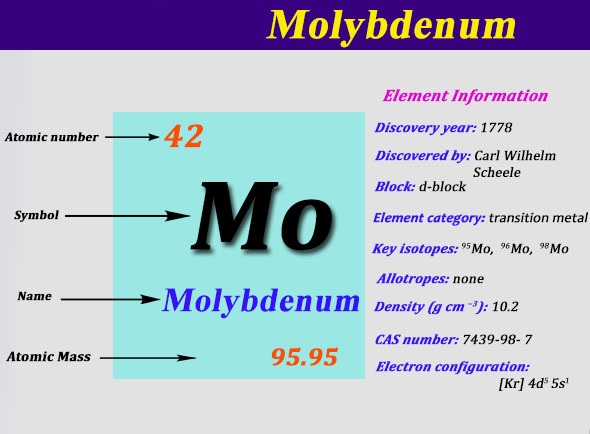
The Electron Configuration of the Molybdenum can be represented as Molybdenum is having the 42 as its atomic number and is located in the group 6 period 5 of the periodic table.
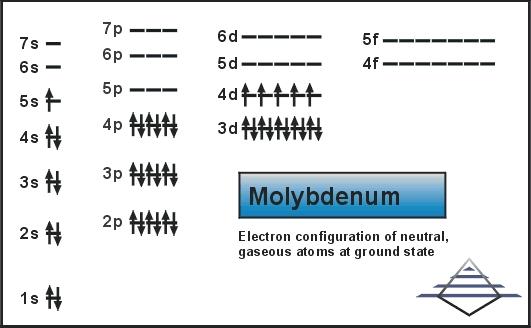
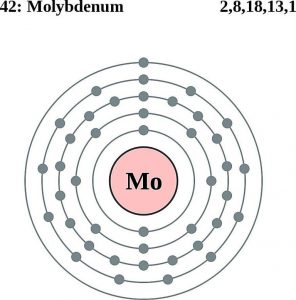
This electron configuration of Mo is in the condensed form and on the ground state. Molybdenum further holds 42 electrons in its shell structure of 2.8.18.13.1
How Many Valence Electrons Does Molybdenum Have?
Molybdenum is the one such element which is having a very soft physical structure and considered to be very ductile. This chemical element is capable of holding the one Electron in the 5S and the further the five Electrons in the 4d shells. This whole structure gives Molybdenum the valence of 0,2+,3+,4+,5+ and 6+.
Molybdenum Number of Valence Electrons
As we have explained above that the Molybdenum is having the Valence of 0,2+,3+,4+,5+ and 6+.
The simple explanation behind this Valence of Molybdenum is the capability of this chemical element. It can use the one Electron in the 5s shell and then it further can use the 5 electrons in the 4d shell due to its extreme flexibility which is unique in its kind.