Argon Electron Configuration: Argon is a chemical element which has a symbol of Ar. Its atomic number is 18. It is in the 18th group of the periodic table and is a noble gas. It is the third most abundant gas in the atmosphere of the earth.
Argon Electron Configuration
It is twice more as abundant as water vapour and 23 times as abundant as CO2 (Carbon dioxide). Also, it is more than 500 times as abundant as neon. This most abundant noble gas comprises 0.00015% of the Earth’s crust.
Almost all of the argon on the Earth’s atmosphere is radiogenic argon-40 which is derived from the potassium-40 decay in the Earth’s crust. Today we are going to provide you with the information about the electron configuration of Ar.
- Manganese Valence Electrons
- iron Valence Electrons
- Cobalt Valence Electrons
- Phosphorus Valence Electrons
- S Valence Electrons
- Cl Valence Electrons
- Nickel Valence Electrons
- Copper Valence Electrons
What Is The Electron Configuration of Argon
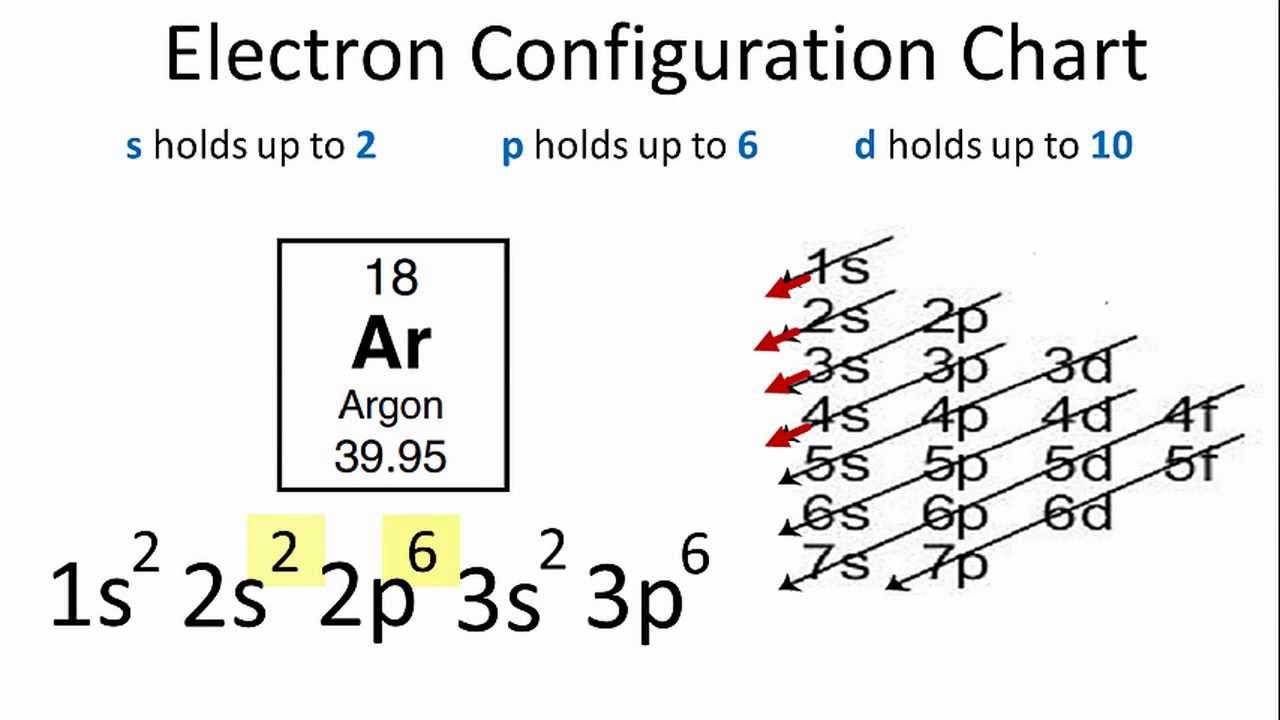
Argon’s electron configuration for the first two electrons goes in the 1s orbital. 1s only hold two electrons and the next 2 electrons for Argon goes in the 2s orbital. The next six electrons go to 2p orbital. The p orbital holds up to six electrons. Hence the Ar electron configuration will be 1s22s22p63s23p6. You can check the periodic table chart for the position of Argon.
How Many Valence Electrons Does Argon Have
Argon has 8 valence electrons in its outer shell.
Argon Electron Configuration Excited State
Electron configuration of Ar is 1s2 2s2 2p6 3s2 3p6

Leave a Reply