Phosphorus Electron Configuration: Phosphorus is a chemical element which has a symbol P. The atomic number of phosphorus is 15. It exists in two major forms, red phosphorus, and white phosphorus, but since it is highly reactive, it is not found as a free element on Earth.
- H Electron Configuration
- He Electron Configuration
- Li Electron Configuration
- Beryllium Electron Configuration
- B Electron Configuration
- C Electron Configuration
- F Electron Configuration
- Neon Electron Configuration
- V Electron Configuration
- Clorine Electron Configuration
Phosphorus Electron Configuration
It is concentrated in the Earth’s crust in about one gram per kilogram. With very fewer exceptions, minerals that contain phosphorus are in the highly oxidized state as inorganic phosphate rocks. Elemental phosphorus was previously isolated in 1669 and a faint glow when it is exposed to oxygen.
Today we will give you all the information about the electron configuration of the phosphorus. Please see the full post for more information.
Phosphorus Number of Valence Electrons
Phosphorus has 5 valence electrons in its outer shell.
Valence Electrons of Phosphorus
There is 5 valence electron in the outer shell of the phosphorus.
What is the Electron Configuration of Phosphorus
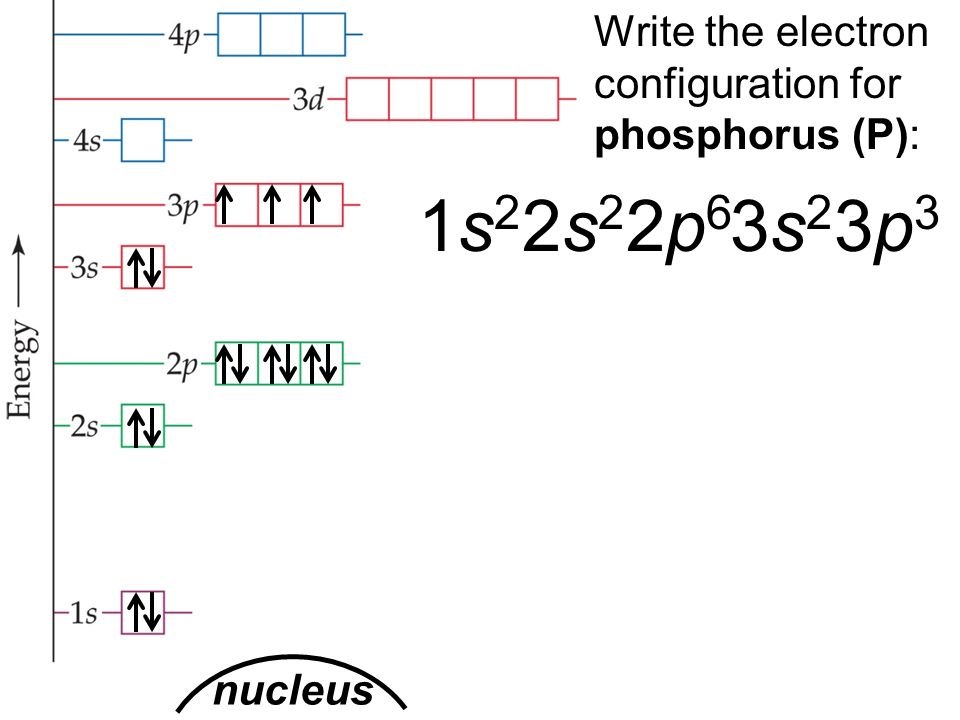
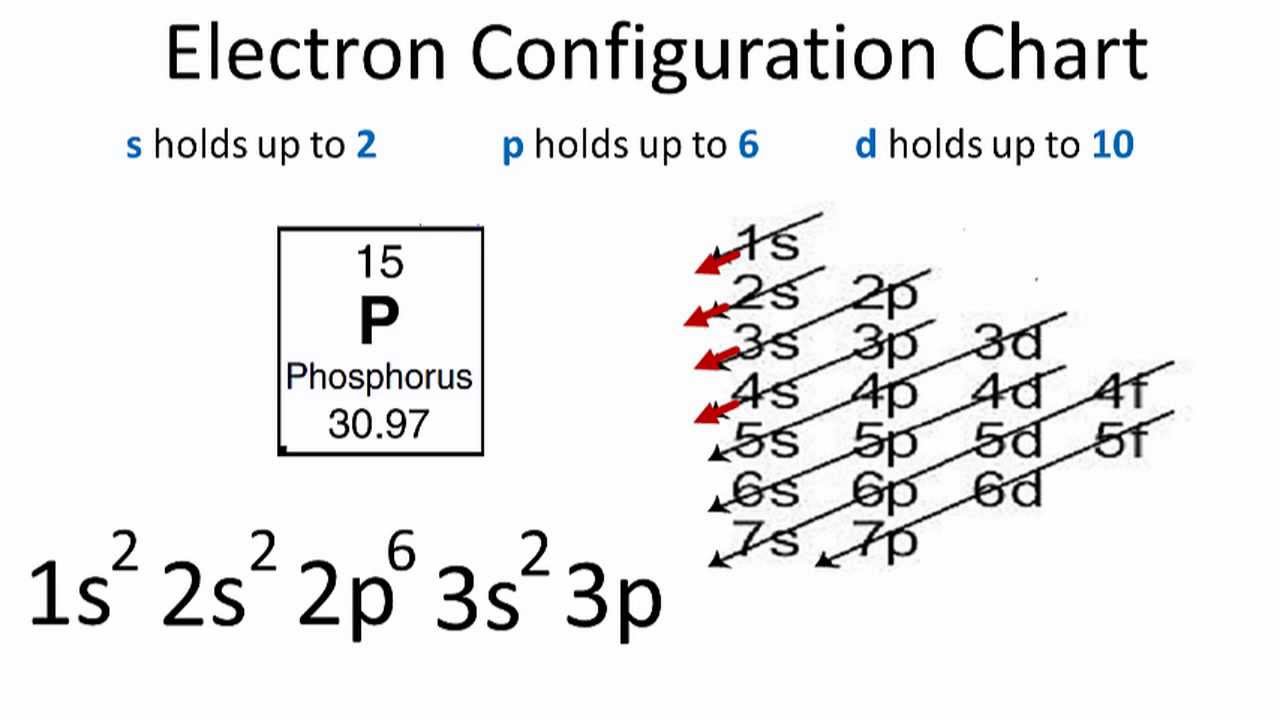
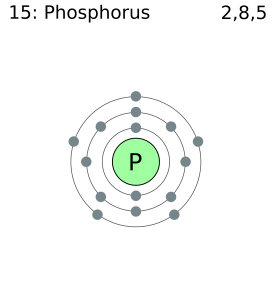
Phosphorous is a chemical element that has an atomic number of 15. Its electron configuration with regards to electrons present in each shell is 2,8,5 and with regards to molecular orbitals, its configuration is 1s2, 2s2 2p6, 3s2 3p3.
How Many Valence Electrons Does Phosphorus Have
According to the periodic table, the phosphorus lies in Group 5A. So, Its valence electrons are 5. The outermost orbitals are 3s2 3p3 which contain 5 electrons.
Leave a Reply