Explore the Gallium Electron configuration here in our article and build a solid understanding of this chemical element. Here in the article, we shall discuss the various chemical properties and the significant usage of the element.
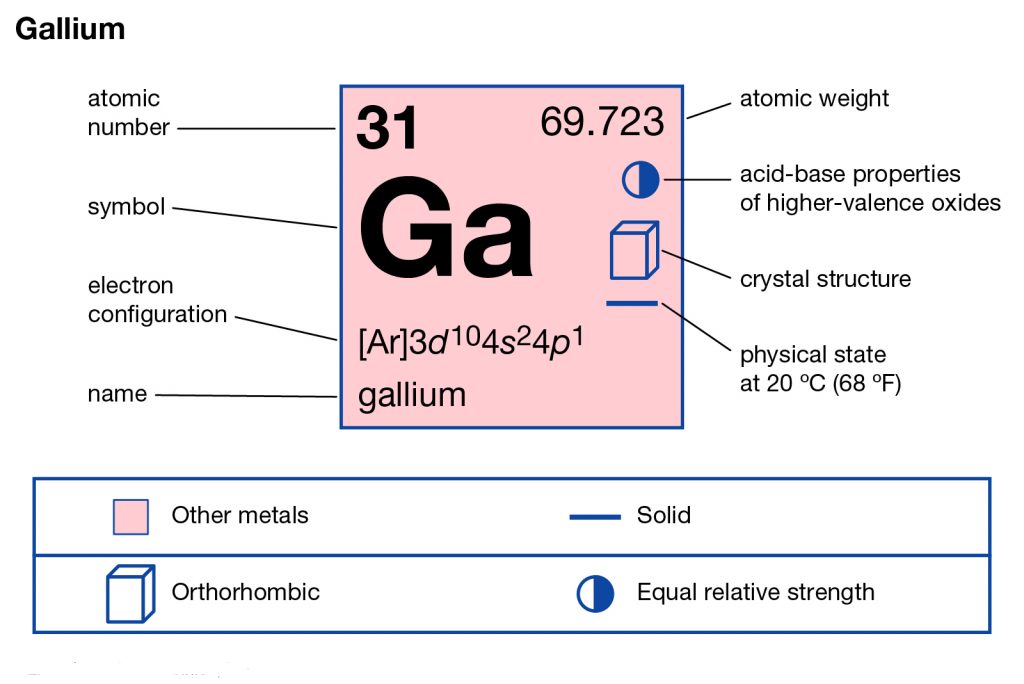
In chemistry, Gallium is the name of a well-known chemical element that has the atomic symbol of 31. It has the symbolic sign of Ga and was discovered by a French scientist back in the year 1875. Gallium is basically the metallic chemical element that falls in group 13 of the periodic table. You can closely relate or associate the chemical element with other metallic elements such as Indium, aluminum, etc.
Gallium Electron Configuration
Gallium basically has no free occurrence form in the earth since it occurs with zinc and bauxite ores. It has the physical texture as the white silvery form that may be available both in the solid and liquid forms. The chemical element however has significant availability around the world as per the respective requirements.
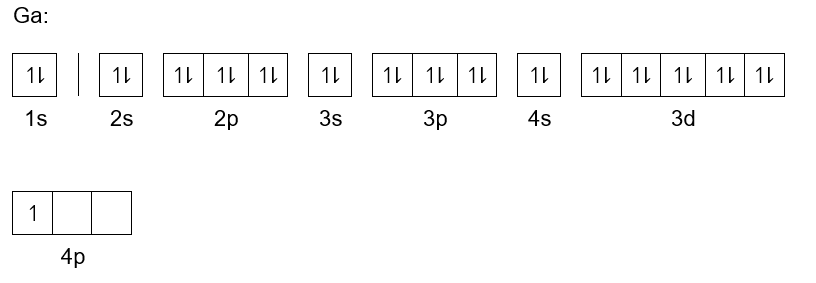
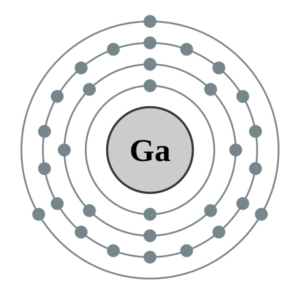
Gallium electron configuration is definitely the subject of discussion for all enthusiasts of chemistry. It’s because the electron configuration is what actually helps in the simplification of the element. It’s actually the process of distributing the electrons of the Gallium to its atomic orbitals. In the electron configuration, we basically break down the whole electrons of the element and then make its proper distribution to the orbitals.
How Many Valence Electrons Does Gallium Have?
The long form of the Gallium electron configuration is 1s22s22p63s23p63d104s24p and you can also convert it to the standard short form. Subsequently, the short form of the electron configuration for the Gallium is Ar 3d10 4s2 4p1. This is the widely known and recognized form of Gallium electron configuration. You can keep both forms in your head to explore the chemical element in depth.
Electron Configuration for Gallium
Well, Gallium being the metallic chemical element has very high relevance across its industrial application. For instance, Gallium is one of the highly usable chemical elements in electronic items. It includes popular electronics such as LEDs, mobile phones, touch sensors, and the list goes on.
The chemical element is also very useful to work as a semiconductor. In other words, Gallium is a perfectly versatile chemical element that can play a number of roles in various domains. The demand for Gallium in the industrial domain is very high and that’s what makes it a highly scalable chemical element. In fact, 90% of the Gallium is useful purely as a semiconductor around the world. So, we believe our article must have been helpful to define the Ga electron configuration and its other important properties.