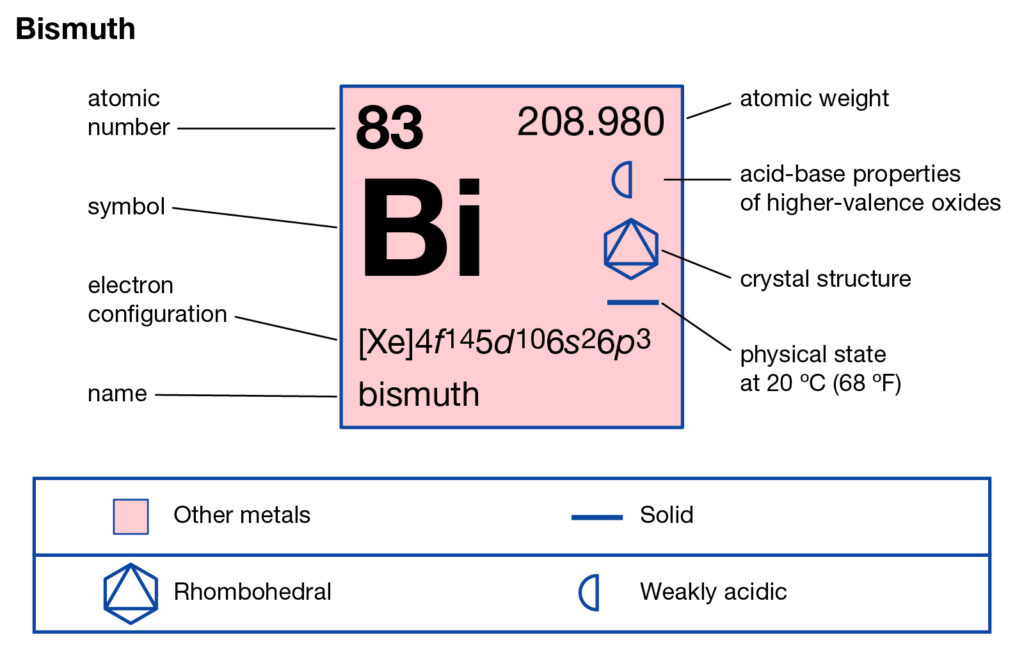
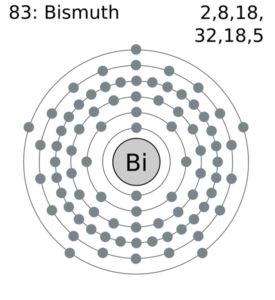
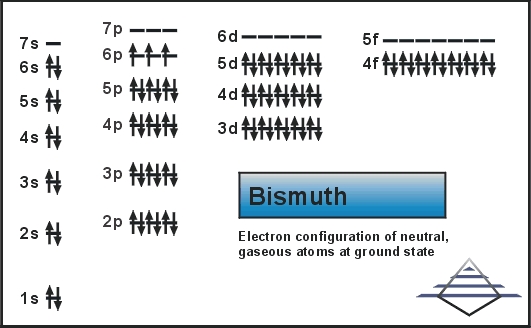
Explore the Bismuth valence electrons here in the article to know the element better. You can also learn about the other important properties of elements. Bismuth is a chemical element coming from the category of high atomic value elements. It has the atomic number 83 and the representative symbol as Bi.
How many valence electrons does Bismuth have?
It belongs to the category of pentavalent post metals. Being the transition metal Bismuth has similar properties as are of Antimony and Arsenic. Bismuth is although available in nature yet the major part of it comes from the Sulfide and Oxide ores. It has the structure closely matching its density to lead by 90% percent.
In the periodic table, Bismuth has diamagnetic properties, however, it has very low thermal conductivity properties. Further, it is a very weak conductor of radioactivity and hence is quite safe. The chemical element is available in the Earth’s crust in a quantity double that the gold. So, Bismuthinite and Bismite are the ores where Bismuth is available.
Australia, China, and Bolivia are the nations having the maximum amount of native Bismuth. The price of Bismuth almost has remained stable over the period of time. Bismuth has very little usage in the commercial domain in the present domain. So, major commercial use of bismuth includes cosmetics, medicines, alloys production etc.
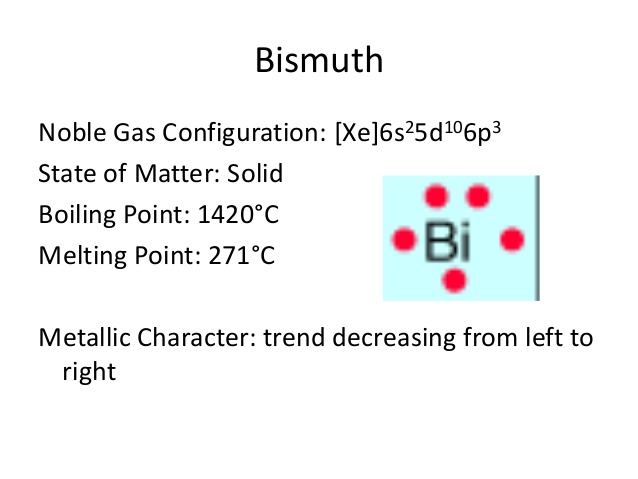
Bismuth Valence Electrons Dot Diagram
Get to understand the Bismuth valence electrons in better manner with the dot diagram. The diagram represents the valence electrons of atoms in the form of dots.
The dots are equivalent to the numbers of valence electrons around the bismuth. Further, the diagram also explains the chemical interactions of valence electrons.
Valency of Bismuth
Bi holds 5 valence electrons in its outer energy shell. So, you can consider the valency of bismuth as 5 for most of its states. Valency is the combining capacity of bismuth to interact with other elements. It’s the noble properties of bismuth to understand by the readers