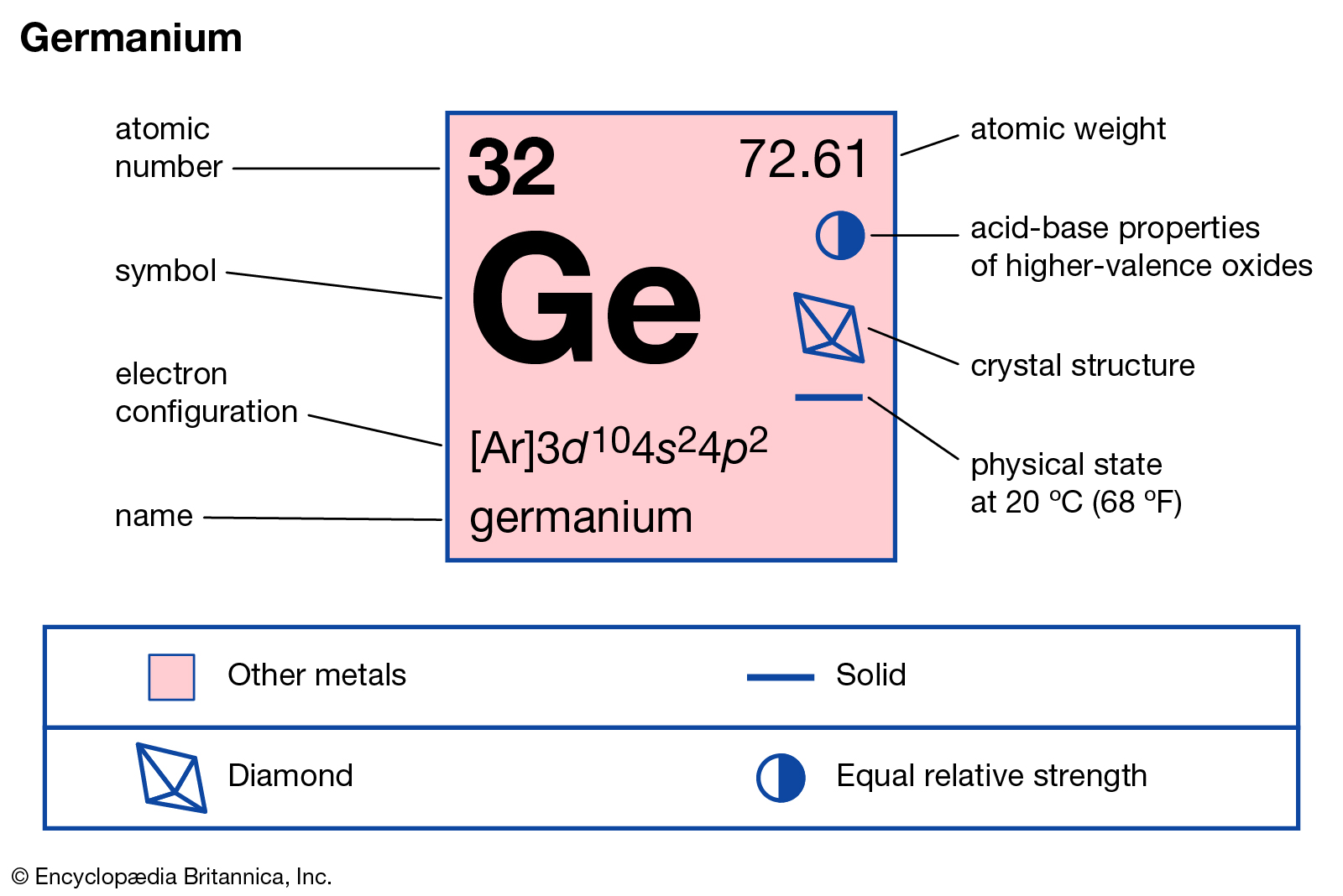
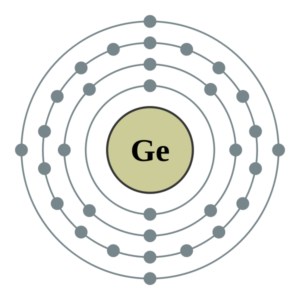
You can here study the Germanium valence electrons to understand this element. We shall also provide the other possible details of this element. Well, you can know the Germanium as the chemical element of chemistry. The element has a fixed atomic number of 32.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Sulfur Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
How many valence electrons does Germanium have?
The chemical element has a brittle grayish metallic form. You can find the Germanium in abundance quantity within the crust of Earth. Germanium is basically the sibling of silicon as they both have similar properties. The pure form of Germanium is highly reactive just like the semiconductors.
Germanium basically has wide usage across the telecom sector around the world. Modern communication cables such as fiber optics, infrared optics are and of this element. Furthermore, semiconductors also have wide usages of Germanium as its key component. Solar panels also use the Germanium as their key component.
Germanium Valence Electrons Dot Diagram
Explore the valence electrons of atoms for Ge with the Lewis dot diagram. It’s a simple yet very effective tool to show the valence electrons Germanium. The diagram basically draws the dots around the representing symbol of Germanium. These dots are exactly the numbers of valence electrons of atoms.
Furthermore, the dot diagram also shows the bonding pattern of valence electrons for Germanium. The single pair of dots depict the single bonding while the double pair depicts the double bonding pattern.
Valency of Germanium
The exact valency of Germanium is four as it holds four electrons in its outer shell. Valency is the combining capacity of the Germanium. The combining capacity of Germanium is relevant in order to attain stability. So, it may either gain or lose four electrons to attain the stable state.
Moreover, you can check the periodic table to cross-check the valency of Germanium. It will help you in getting the other information as well about the Germanium.