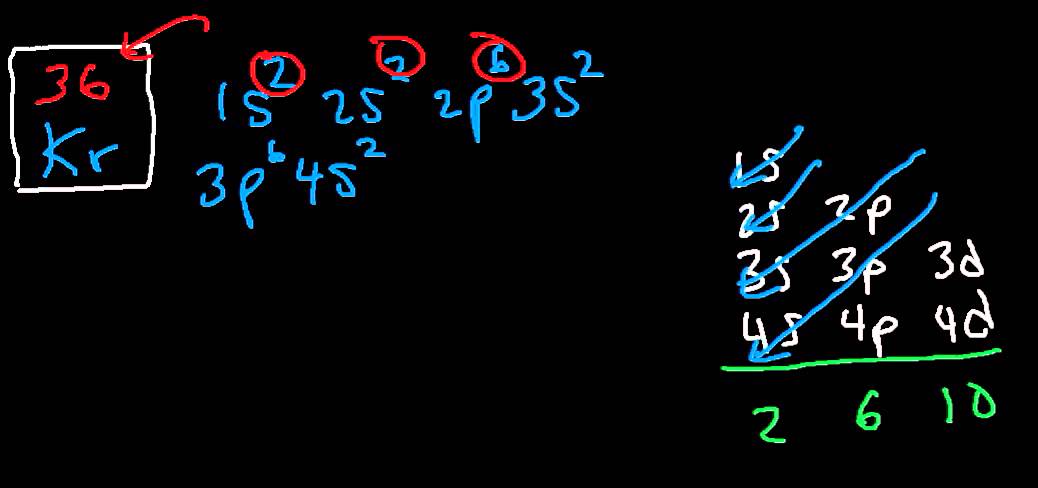Krypton Electron Configuration: Krypton is a chemical element that has a chemical symbol Kr. The atomic number of Krypton is 36. It is a member of group 18 of the periodic table. It is an odorless, colorless, tasteless noble gas. It occurs in a little amount in the atmosphere. It is sometimes used with other rare gases in fluorescent lamps. With some of the rare exceptions, Chemically Krypton is inert.
Like the other noble gases, Krypton is also used in photography and lighting. Light of Krypton has many spectral lines, and krypton plasma is used in high-powered gas, bright lasers each of which amplifies and resonates a single spectral line. It also makes a useful laser. Today we are are going to share the information about Electron configuration for Krypton.
- Cobalt Electron Configuration
- Nickel Electron Configuration
- Copper Electron Configuration
- Chlorine Electron Configuration
- Argon Electron Configuration
- Potassium Electron Configuration
- Scandium Electron Configuration
- Titanium Electron Configuration
- Vanadium Electron Configuration
- Nickel Electron Configuration
- Zinc Electron Configuration
- Germanium Electron Configuration
- Arsenic Electron Configuration
- Selenium Electron Configuration
- Bromine Electron Configuration
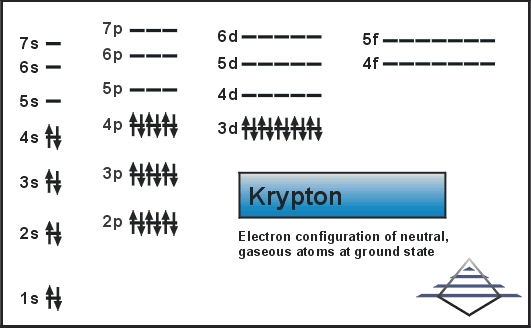
What is the Electron Configuration of Krypton
[Ar] 3d10 4s2 4p6 is the electron configuration of Krypton.
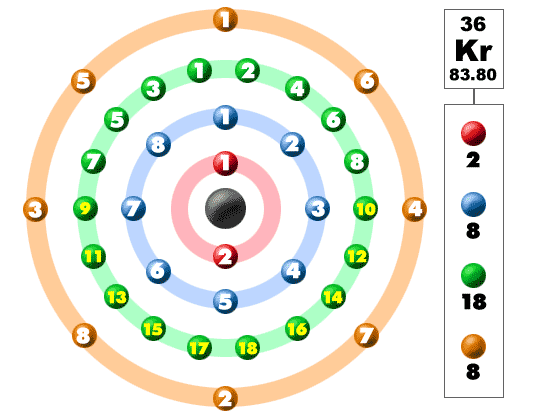
How Many Valence Electrons Does Krypton Have
There are eight valence electrons in the outer shell of Krypton. You can see these valence electrons in the picture which is provided above.
Krypton Number of Valence Electrons
Krypton has eight valence electrons in its outer shell.