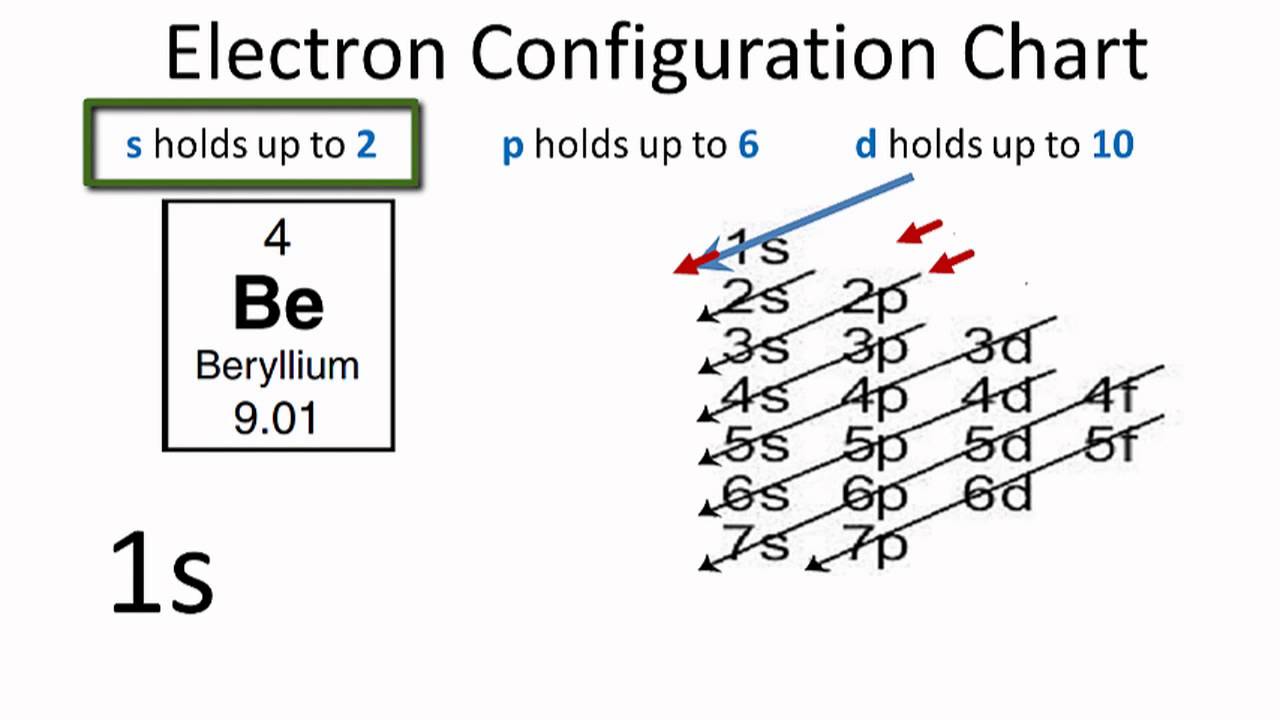
Beryllium Electron Configuration: Beryllium is the fourth atomic number of the periodic table. The symbol of Beryllium is “Be” and it is a very rare element which is found in the universe. Usually, this element occurs as the product of the separation of the larger atomic nuclei which are collided with the cosmic rays.
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
Beryllium Electron Configuration
Electronic configuration can be defined as the distribution of the electrons in the atomic shells of the atoms or molecules. In the case of Beryllium, there are 4 electrons that are present in the 2 orbits of its atom.
The electronic configuration of Beryllium is: 1s22s2 or can also be represented as [He] 2s2.
Also, check here the Hydrogen Electron Configuration
What is The Beryllium Electron Configuration
The electronic configuration of beryllium is the distribution of its 4 electrons in the shells of the atom and it can be represented as 1s22s2
How Many Valence Electrons Does Beryllium Have
Valence electrons can be defined as the number of electrons present in the outermost shell of the atom. In the case of beryllium, there are 2 electrons present in the second or outermost shell. So, the number of valence electrons of beryllium are 2.
Beryllium (Be) Number of Valence Electrons
The number of valence electrons in beryllium is 2. As the electrons located in the outermost orbit is considered as valence electrons and beryllium has 2 electrons in its outer shell.