Get the proper knowledge of Chromium valence electrons here in the article. We will talk about the other various characteristic of the element. Chromium is a chemical element as in the language of chemistry. It’s one of those elements which have little to no naturally occurring sources.
- Flerovium Valence Electrons
- Plutonium Valence Electrons
- Mercury Valence electrons
- Neptunium Valence Electrons
- Moscovium Valence Electrons
- Cesium valence electrons
- Bismuth Valence electrons
- Silicon Valence Electrons
- Livermorium Valence Electrons
- Radon Valence electrons
- Xenon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Hydrogen Valence Electrons
- Phosphorus Valence Electrons
- Helium Valence Electrons
- Lithium Valence Electrons
- Americium Valence Electrons
- Beryllium Valence Electrons
- Boron Valence Electrons
- Sodium Valence Electrons
- Carbon Valence Electrons
- Nitrogen Valence Electrons
- Aluminum Valence Electrons
- Oxygen Valence Electrons
- Iodine Valence Electrons
- Fluorine Valence Electrons
- Magnesium Valence Electrons
- Neon Valence Electrons
- Sulfur Valence Electrons
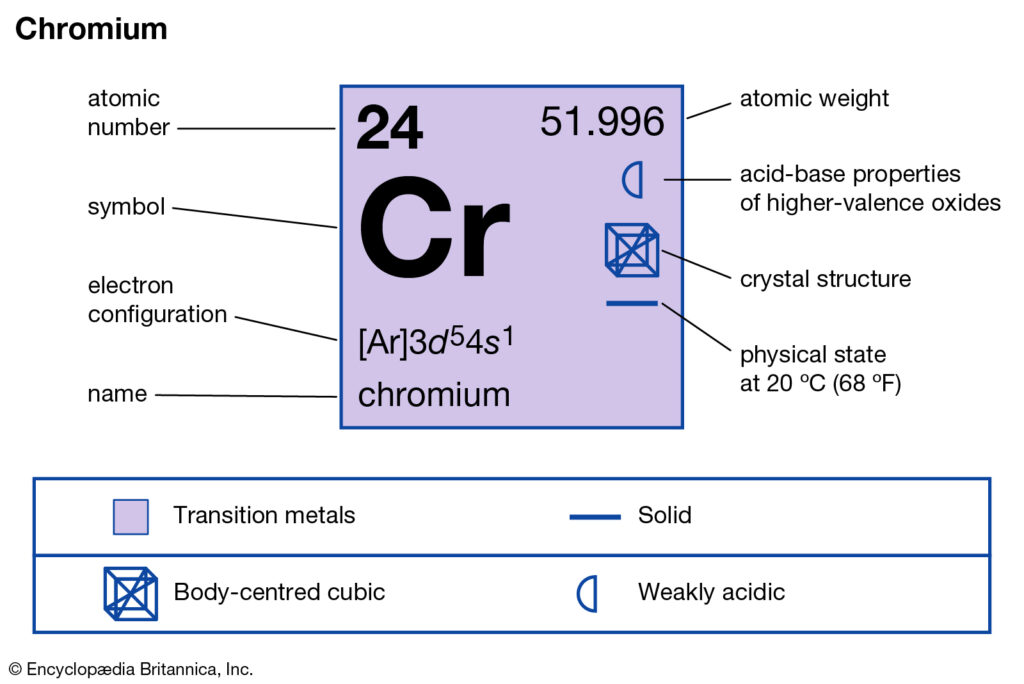
How many valence electrons does Chromium have?
The chemical element belongs to the group 6 category of the periodic table. It has the atomic number as 24 and the representative symbol as Cr. Chromium is quite a popular metal element across its industrial usage. It’s the perfect additive to steel and hence it works generally in integration.
Presently there are many steel tools, which are made in integration with the Chromium. The chemical element is quite strong and reliable in its usage. Furthermore, chromium is highly capable of being used as polished metal. This is why the manufacturing industry quite in awe of the metal.
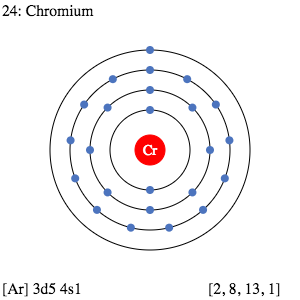
Chromium Valence Electron Dot Diagram
You can get a better insight into Chromium valence electrons with the dot diagram. We call the dot diagram the Lewis dot diagram too of valence electron.
With the help of a dot diagram, you can figure out the valence electrons of the atom in the molecule. Furthermore, you can also figure out whether valence electrons exist in pairs or in bonding. If the valence electron exists in a single bond then the pair of dots represents it. The double pair of dots shows the double bonding of electron valency of atoms.
Valency of Chromium
Well, the valency of Chromium may be either of 2 or 3 as per the prevailing state. The valency of Chromium varies because it’s a transition state. Being the transition element, it is capable of exhibiting valency. Furthermore, Chromium is the element of d block which shows similar characteristics.