Learn everything about Gallium valence electrons here and get a step further with your chemistry knowledge. You will also learn about the other details of this element. Gallium is a chemical element in the field of chemistry. It belongs to the p block category of the periodic table.
- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Moscovium Valence Electrons
- Sodium Valence Electrons
- Cesium valence electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Sulfur Valence Electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
- Nitrogen Valence Electrons
- Phosphorus Valence Electrons
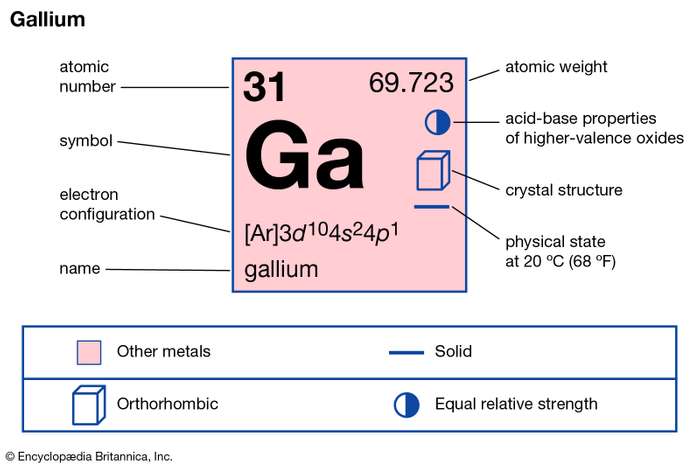
How many valence electrons does Gallium have?
Gallium has the atomic number 13 and the representative symbol as Ga. It’s basically a soft silvery metal in its appearance. Gallium belongs to the family of Aluminum, thallium, indium. It has no free availability in nature, as it occurs within the bauxite and zinc ores.
Just like other chemical elements Gallium also has significant applications. The purest form of Gallium is highly useful in the production industries. Gallium has a significant contribution in the production of semiconductors. Further, in a similar way Gallium is useful in various electronic products.
In the US more than 65% of semiconductors are made of pure gallium. It is also useful in the transmission of cable tv, satellite, wireless infrastructure, etc.
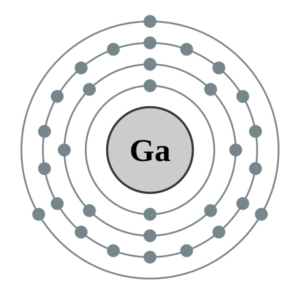
Gallium Valence Electrons with Dot Diagram
Lewis dot diagram is a very useful tool to figure out the Valence electrons of atoms in Gallium. The diagram uses the simple dots around the element to depict the numbers of electrons. You can possibly draw the dot diagram for all types of chemical elements including gallium. The diagram breaks out the electron configuration of the element to conclude its valence electrons.
Furthermore, the Dot diagram is also useful in understanding the type of bonding pattern of valence electrons. It provides details on whether the valence electrons take place in single or double bonding.
Valency of Gallium
The exact valency of Gallium is 3 with its corresponding compounds. We also know the valency as the combining capacity of a given element.
Valency is generally the number of electrons in the outermost shell of Gallium. Valency helps in understanding the true character of the chemical elements.
Leave a Reply