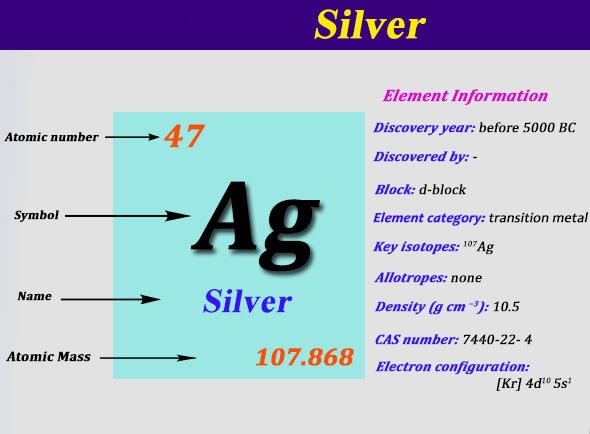
Electron Configuration For Silver: Silver is a chemical element that has a chemical symbol, Ag. The atomic number of silver is 47. It is a white, soft, lustrous transition metal. Silver exhibits the highest thermal conductivity, electrical conductivity, and reflectivity of all metals.
- Bromine Electron Configuration
- Sodium Electron Configuration
- Magnesium Electron Configuration
- Aluminum Electron Configuration
- Silicon Electron Configuration
- Sulfur Electron Configuration
- Chlorine Electron Configuration
- Phosphorus Electron Configuration
- Argon Electron Configuration
- Potassium Electron Configuration
Electron Configuration For Silver
It is found in the Earth’s crust in the free and pure elemental form in the form of an alloy with gold and other metals, and also in minerals like chlorargyrite and argentite. Silver is mostly produced as a byproduct of gold, copper, zinc and lead refining.. It has been valued as a precious metal for a very long time.
This metal is used in many bullion coins, and also sometimes alongside gold. It is much less abundant as a native metal while it is more abundant than gold. The purity of silver is typically measured on a per-mile basis. The 94%-pure alloy is described as 0.940% Pure.
Silver has had an enduring role in most human cultures as one of the seven metals of antiquity. Other than an investment and as a currency medium ( bullion and coins), It is also used in water filtration, solar panels, ornaments, jewelry, high-value tableware, and utensils.
What Is The Electron Configuration of Ag?
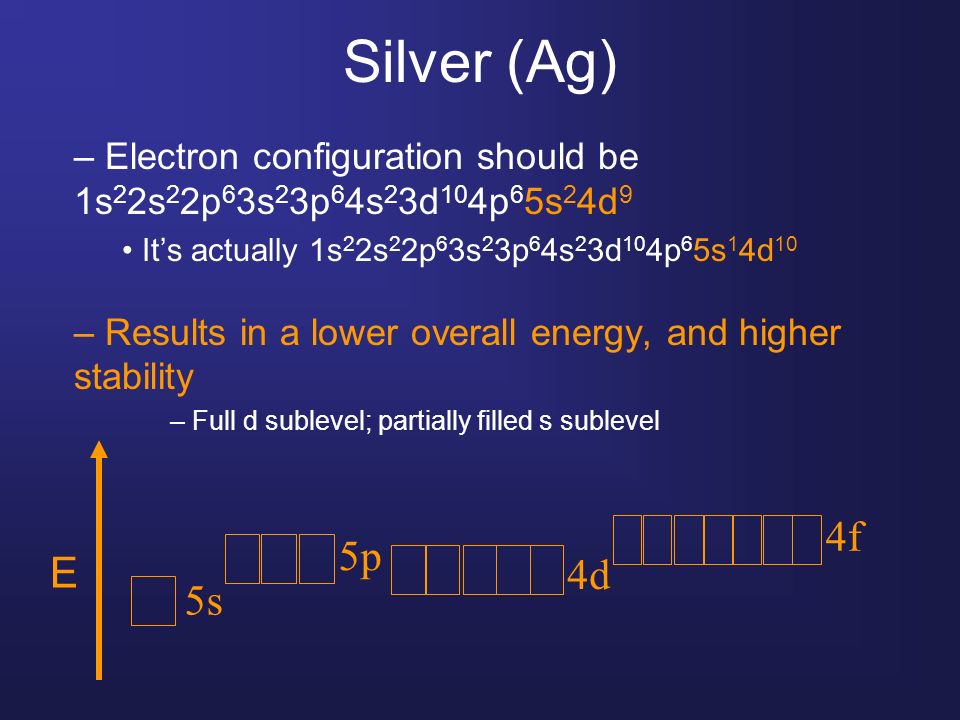
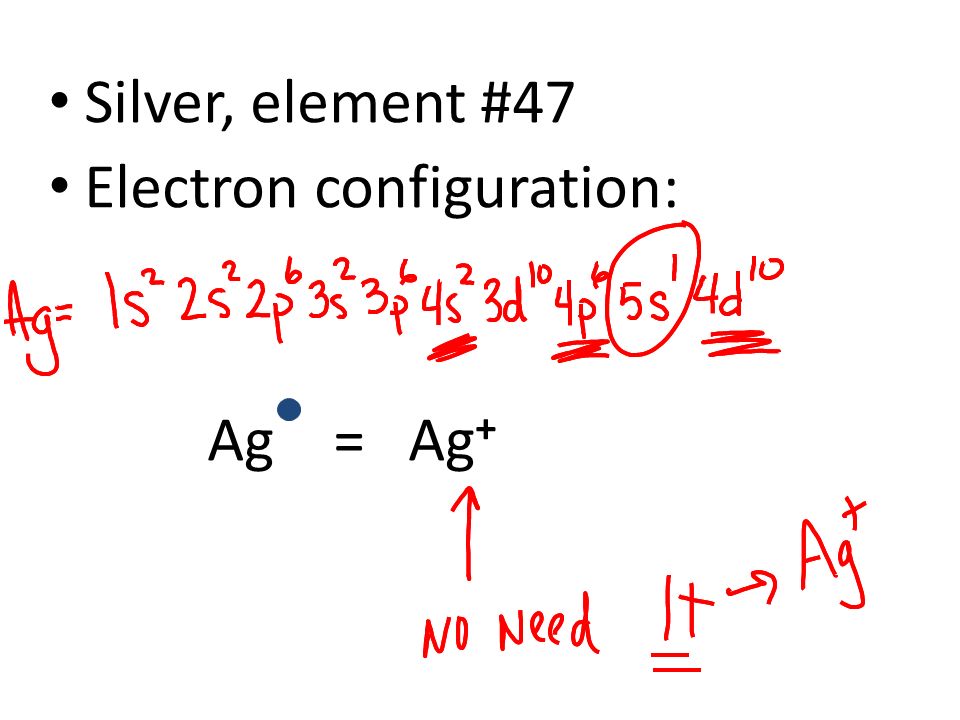
The electron configuration for silver (Ag) depends upon the location meant for silver in the fifth row and eleventh column of the periodic table. That is why the electron configuration for (Ag) silver should end as 4d9, and hence it is 1s22s22p63s23p64s23d104p65s24d9
How Many Valence Electrons Does Silver Have
Silver has only one valence electron in its outer shell. See the picture below for more information.
Silver Number of Valence Electrons
There is only one valence electron in the outer shell of the Silver. For more information, you can also refer to the pictures provided in this post.
Leave a Reply