Rubidium Electron configuration is basically the distribution of electrons in the orbital of molecules. This electron configuration is generally made taking any chemical element and today in this article we are going to discuss the Electron configuration of Rubidium.
Rubidium Electron Configuration
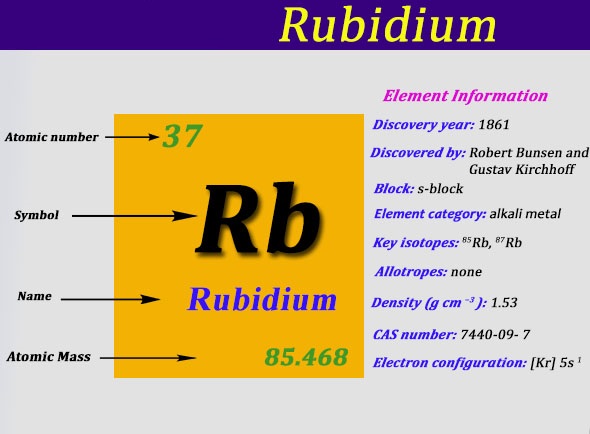
Rubidium is basically a chemical element which was first discovered in the year of 1861. The Rubidium chemical element is symbolized by the Rb abbreviation and it holds its atomic number as 37.
The electron configuration of Rubidium simply implies making the distribution of Rubidium’s electron in the molecular orbital. Rubidium is considered to be the highly reactive element and it reacts very violently with the water causing fire consequently.
- Cobalt Electron Configuration
- Nickel Electron Configuration
- Copper Electron Configuration
- Chlorine Electron Configuration
- Argon Electron Configuration
- Potassium Electron Configuration
- Scandium Electron Configuration
- Titanium Electron Configuration
- Vanadium Electron Configuration
- Nickel Electron Configuration
- Zinc Electron Configuration
- Germanium Electron Configuration
- Arsenic Electron Configuration
- Selenium Electron Configuration
- Bromine Electron Configuration
What is The Electron Configuration of Rubidium?
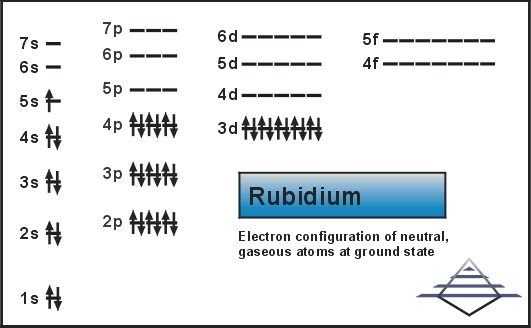
The electron configuration of Rubidium can be written as [Kr] 5s1 and here is the simple explanation behind this configuration. We know that the atomic number of the Rubidium is 37, and further the Kr has the 36 atomic number hence we get only 1 electron from Rubidium which is to be assigned in the orbital.
As the Kr is the last element in the fourth periodic table and with that theory, the remaining 1 electron of the Rubidium will become 5. Now the S sub-shell will be filled first and this whole distribution will be written as
How Many Valence Electrons Does Rubidium Have?
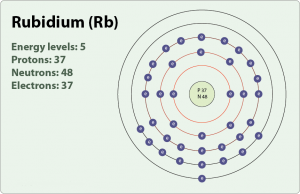
Rubidium holds one valence of the electron which is held in the S orbital. Rubidium is the chemical element with the 37 electrons and is represented by the Rb in the chemical periodic table. Rubidium comes in the category of the Alkali metals which always hold one valence of the electron.
Rubidium Number of Valence Electron
Rubidium element holds the 37 electrons and it has the 1 valence of the electron in the S orbital. Kr has the 36 atomic number and due to that, there is only one 1 electron in the Rubidium’s valence shell.
Leave a Reply