Lithium Electron Configuration: Li element is also known as a Li salt. They are basically used for psychiatric medication. This also includes the major depressive disorder treatment which does not improve following the use of other antidepressants, and bipolar disorder. In such disorders, the risk of suicide gets reduced.
Lithium is a chemical element that has the symbol Li and its atomic number is 3. It is a silvery-white and soft alkali metal. Under certain situations, it is the lightest solid and lightest metal element. Like all alkali metals, it is a highly flammable and reactive metal. It is stored in mineral oil. When it is cut open, it shows a metallic luster. See the full post below to know the electron configuration of the Li. Check here the complete periodic table.
- Oxygen Electron Configuration
- Fluorine Electron Configuration
- Neon Electron Configuration
- Thorium Electron Configuration
- Protactinium Electron Configuration
- Neptunium Electron Configuration
- Plutonium Electron Configuration
- Americium Electron Configuration
- Nobelium Electron Configuration
- Gold Electron Configuration
- Mercury Electron Configuration
- Flerovium Valence Electrons
- Moscovium Valence Electrons
- Livermorium Valence Electrons
- Tennessine Valence Electrons
- Oganesson Valence Electrons
- Neptunium Valence Electrons
- Plutonium Valence Electrons
- Americium Valence Electrons
- Antimony Valence Electrons
- Tellurium Valence Electrons
- Iodine Valence Electrons
- Xenon Valence Electrons
- Caesium Valence Electrons
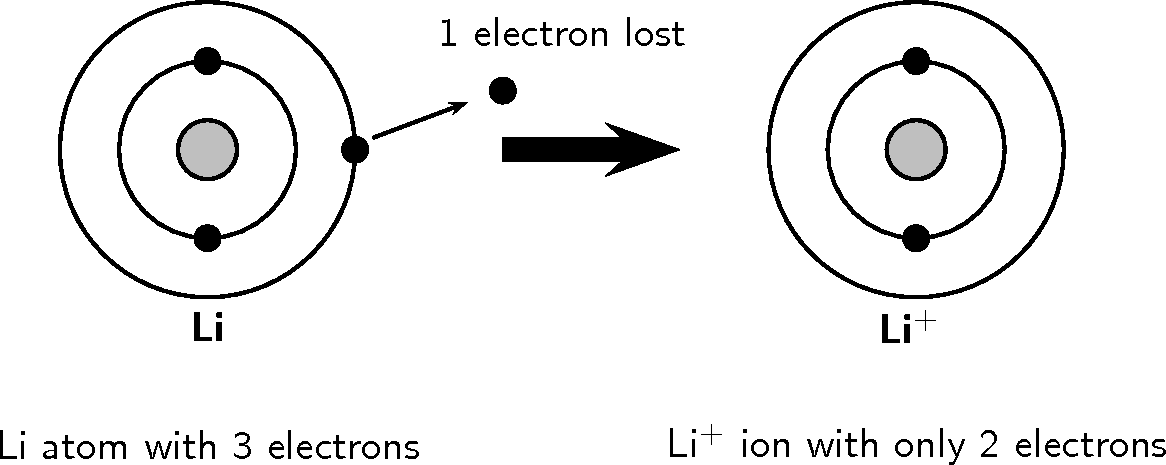
Electron Configuration For Li-Ion
The electron configuration of lithium-Ion is [He] 2s1.
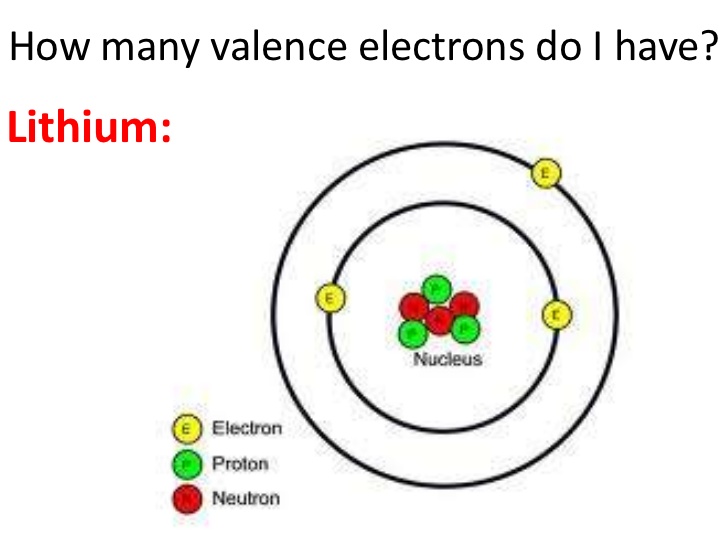
Lithium Number of Valence Electrons
Lithium has only one valence electron.
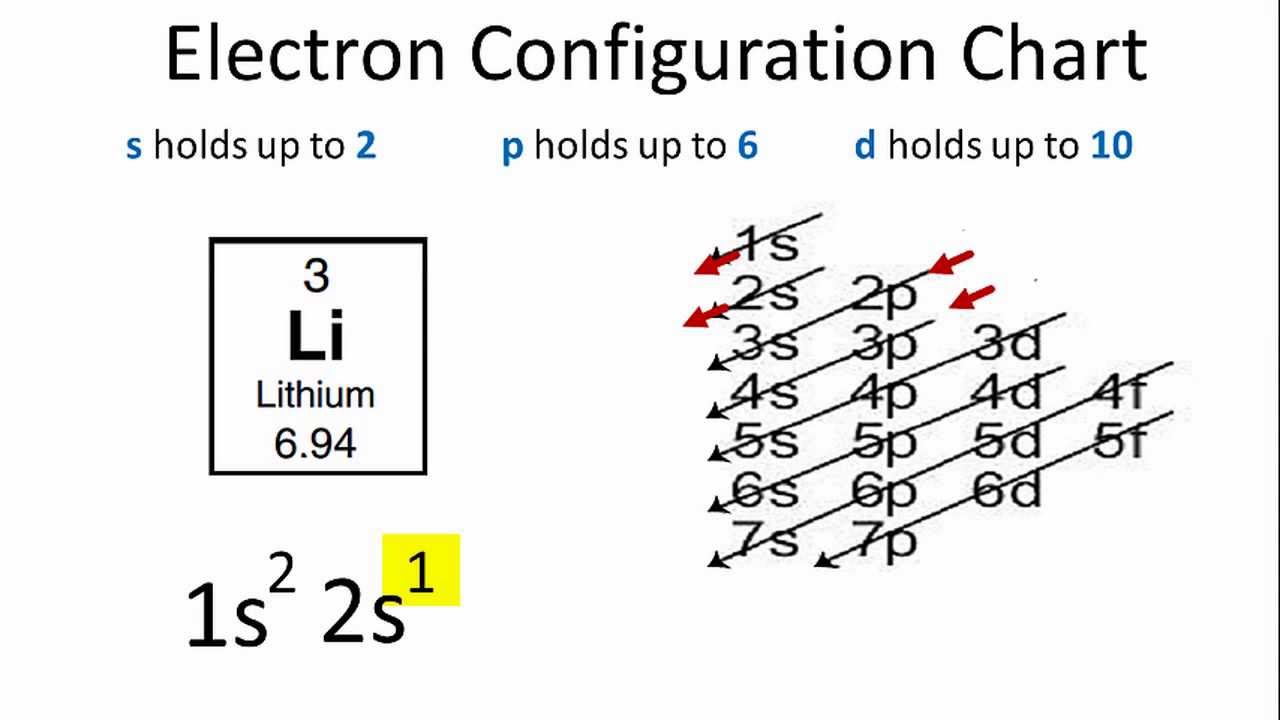
Lithium Electron Configuration (Li)
How Many Valence Electrons Does Lithium Have
Only one valence electron is found in the Li. You can see the image below.
What is the Electron Configuration of Li
See the image below to know the electron configuration of Li.
Leave a Reply